SAN MATEO, CA—The U.S. Food and Drug Administration (FDA) has cleared the SoundBite Hearing System for use in treating patients with conductive hearing loss. SoundBite, which is manufactured by Sonitus Medical, Inc., a San Mateo medical device company, is the first non-surgical and removable hearing system to transmit sound via the teeth.
Earlier this year, the FDA had cleared SoundBite for treatment of patients with single-sided deafness. Approval for use with conductive hearing loss further establishes SoundBite as a non-surgical and less expensive alternative to bone-anchored implants
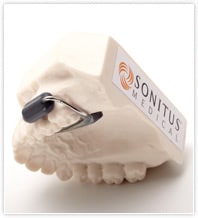
SoundBite imperceptibly transmits sound via the teeth to help people with single-sided deafness or conductive hearing loss who are not candidates for conventional air-conduction hearing aids. It uses bone conduction to deliver sound to the inner ear.
The system consists of an easily inserted in-the-mouth hearing device—custom made to fit around either the upper left or right back teeth—and a small microphone unit worn behind the ear. No modifications to the teeth are required.
The SoundBite hearing system will be available starting early this fall through physicians and audiologists in selected markets in the U.S. It will then be introduced to additional markets nationwide over the following several months. For information on availability, go to www.soundbitehearing.com/find/.
Commenting on the FDA’s action, Amir Abolfathi, CEO of Sonitus Medical, said, “We are pleased that the uses of the SoundBite system continue to expand. As we begin the roll out of our product in partnership with otologists, ENTs, and audiologists, we remain committed to increasing the number of patients who may benefit from our non-surgical treatment option.”