OTTAWA, ONTARIO — Clearwater Clinical Limited announced this week that the company has received CE Mark approval for SHOEBOX Audiometry in Europe “as an audiometer intended for diagnosis of human hearing loss”. With the approval, SHOEBOX becomes the world’s first CE Marked iPad-based audiometer. The device is already listed as a Class II medical device with US Food & Drug Administration (FDA) and Health Canada.
With the CE Mark approval, SHOEBOX can now be used to perform clinical hearing tests in the European market.
“We are proud of this significant milestone. There has been growing demand for SHOEBOX outside of North America. The addition of the CE Mark to our long list of approvals, accreditations, and validations is vital to our planned expansion into the European market. It is also further evidence of our commitment to meeting the highest standards of quality and regulatory compliance as a medical device manufacturer.” –Michael Weider, CEO of Clearwater Clinical
Made up of the SHOEBOX Audiometry software, an iPad, calibrated transducers and a cloud-based data management system, SHOEBOX offers mobile clinicians a highly portable audiometric system.
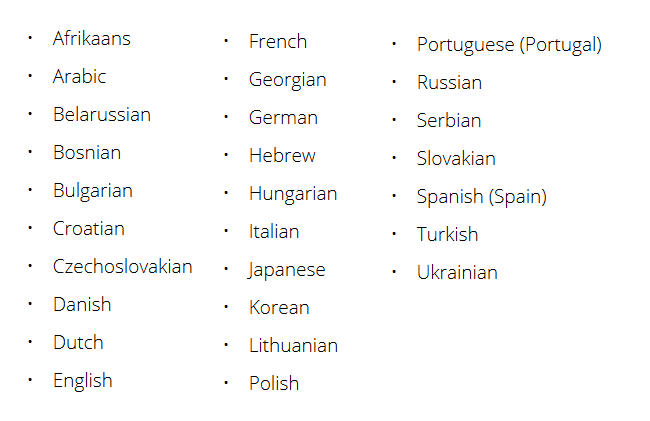
According to the company press release, all patient-facing screens of SHOEBOX Audiometry in automated mode are now available in 27 different languages. Translations include:
https://youtu.be/rFHuYbtVoQU
Source: Clearwater Clinical







