In 2012, I reported here that meta-analysis demonstrated no benefit in reducing the frequency or severity of Meniere’s episodes when compared to placebo. Since that time, one particularly high quality clinical trial and one updated meta-analysis have been published.
First, the updated meta-analysis: A group out of China reviewed multiple studies and did not state that there was no benefit, but certainly did not give the Meniett a ringing endorsement. Here are the last few lines from their publication, “This study has some limitations. First, the included studies may suggest that Meniett therapy might be effective but the conclusion was significantly restricted by the overall lack of control groups and the apparently shortened follow-up period. Second, most of the included studies were retrospective and had small sample sizes. Third, few of the included reports provided detailed information about severity of the disease, and we were unable to identify the impact of disease severity on the prognosis. Fourth, previous endolymphatic sac surgery or intratympanic gentamycin perfusion and bilaterality of the disease may confound the evaluation.
Finally, our meta-analysis showed that: 1) Meniett therapy may prevent vertigo attacks and substantially reduce its frequency in MD patients; 2) the impact of Meniett therapy on hearing remains uncertain; 3) Meniett therapy may alleviate the functional deficit and improve the quality of life; and 4) the optimal effect of Meniett therapy might last for approximately 18 months. In conclusion, it seems that Meniett therapy is, to some extent, effective for the treatment of MD, and may serve as a second-line treatment.”
Next, a new, independent study:
Researchers from Europe (France and Italy) performed a randomized, double blind, placebo controlled study with the goal of determining whether the Meniett device reduced the number of episodes of vertigo associated with intractable Meniere’s disease, and whether there was any noted impact on daily life.
Patients meeting the study criteria were randomly placed into two groups. One group received recommended Meniett treatment, while the second group received a placebo treatment. Patients in both groups showed improvement, which may have been the result of other medical treatment, or possibly a result of the natural course of the disease. No differences were noted between the two groups at the completion of the treatment regimen.
While it is hard to make definitive statements about the effectiveness of treatments for a fluctuating and mysterious disease like Meniere’s disease, the evidence keeps building that the Meniett device is a plausible idea, with little evidence of clinical effectiveness.



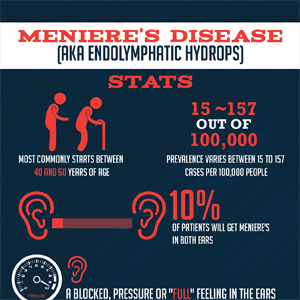



Let’s not forget that a diagnosis of Meniere’s disease is one of exclusion and the cause is unknown (i.e., idiopathic). As a result, it’s no surprise that various treatments for MD may work for some patients but not for others since the underlying pathology may vary considerably across this population.
Thanks for this interesting series of posts, Alan!