PARIS, FRANCE — Researchers have announced the successful restoration of hearing in an adult mouse model of DFNB9 deafness — one of the most frequent cases of congenital, genetic deafness.
Individuals with DFNB9 deafness are profoundly deaf due to a deficiency in the gene coding for otoferlin, a protein which is essential for transmitting sound information at the auditory sensory cell synapses. Through intracochlear injection of this gene in an adult DFNB9 mouse model, the researchers were able to restore auditory synapse function and hearing thresholds to a near-normal level.
These findings, which were recently published in the journal PNAS, “open up new avenues for future gene therapy trials in patients with DFNB9”.
New Treatment Options for Genetic Deafness?
Over half of nonsyndromic profound congenital deafness cases are said to have a genetic cause, and most (~80%) of these cases are due to autosomal recessive forms of deafness (DFNB). Cochlear implants are currently the only option for recovering hearing in these patients.
Adeno-associated viruses (AAVs) are among the most promising vectors for therapeutic gene transfer to treat human diseases. AAV-based gene therapy is a promising therapeutic option for treating deafness but its application is limited by a potentially narrow therapeutic window.
“In humans, inner ear development is completed in-utero and hearing becomes possible at approximately 20 weeks of gestation. In addition, genetic forms of congenital deafness are generally diagnosed during the neonatal period. Gene therapy approaches in animal models must therefore take this into account, and gene therapy efficacy must be demonstrated following a gene injection when the auditory system is already in place. In other words, therapy must reverse existing deafness.”
The team led by Saaïd Safieddine, a CNRS researcher in the Genetics and Physiology of Hearing Unit (Institut Pasteur/ Inserm) and coordinator of the project, used a mouse model of DFNB9, a form of human deafness that represents 2 to 8% of all cases of congenital genetic deafness.
A single intracochlear injection of the vector pair in adult mutant mice was used to reconstruct the otoferlin coding region by recombining 5′ and 3′-end DNA segments, leading to long-term restoration of otoferlin expression in the inner hair cells, and then restored hearing.
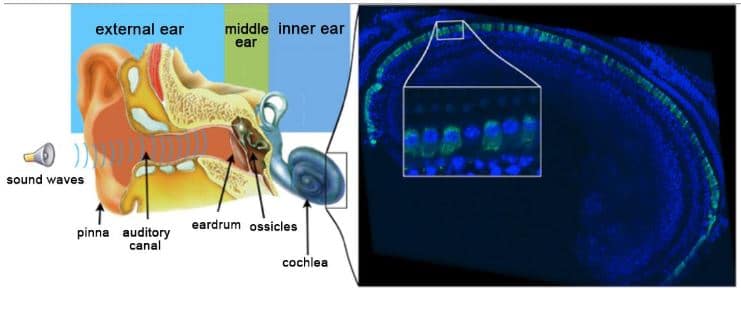
The left panel shows a schematic representation of the human ear. The right panel shows an immunofluorescence image of the auditory sensory epithelium within an injected cochlea. The inner hair cells have been stained for otoferlin in green. Otoferlin is detected in almost all of these cells. The inset is a high magnification area showing an inner hair cell that has not been transduced. Image © Institut Pasteur
The outcomes achieved by the scientists suggest that the therapeutic window for local gene transfer in patients with DFNB9 congenital deafness could be wider than thought, and offers hope of extending these findings to other forms of deafness. These results are the subject of a patent application filed.
Source: Insitut Pasteur





