New research provides unprecedented insight into the complex molecular events underlying hearing at connections between sensory cells and neurons in the inner ear.
The work, conducted by scientists at the University Medical Center Göttingen, advances understanding of sound signal transmission and points to links between synapse function and common forms of hearing loss.
“We aimed to closely replicate physiological conditions to elucidate signaling events critical for hearing. Observing neurotransmission at individual synapses enabled us to quantify key details of the conversion of sound into neural code.”
–Dr. Lina María Jaime Tobón, study lead author
The senses rely on communication between specialized sensory cells and connecting neurons at contact points known as synapses. At these junctions, influx of calcium ions triggers the release of chemical messenger molecules called neurotransmitters, which activate the adjoining neuron.
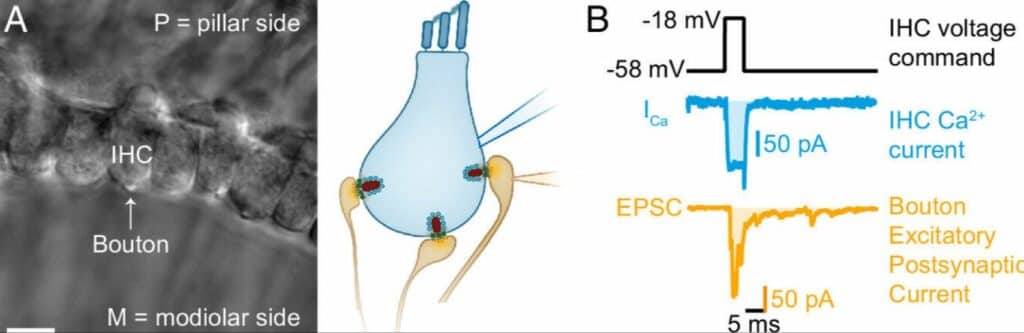
Paired IHC- bouton patch- clamp recordings to study the biophysical properties of individual IHC ribbon synapses. (A) Differential interference contrast (DIC) image of an explanted mouse organ of Corti. In this example, supporting cells from the modiolar side (M) were removed to gain access to the IHCs and their contacting boutons. (B) Evoked release was recorded using depolarizing pulses (black trace), triggering whole cell IHC Ca2+ influx (ICa, blue trace), and concomitant release of neurotransmitter (detected as postsynaptic EPSC, orange trace). Ca2+ charge and EPSC charge were estimated by taking the integrals of the currents (shaded light blue and light orange areas). Image credit PNAS; Tobon & Moser (2023)
The new research, published in PNAS, focused on synapses between sensory hair cells and auditory nerve fibers in the cochlea in the inner ear. Hair cells translate sound-evoked mechanical vibrations into electrical signals. This change in electrical potential drives entry of calcium ions through channels at the synapse, eliciting glutamate release onto the auditory neuron on the other side.
“For the first time, we resolved signaling events enabling hair cells to convert sound information into glutamate release at the speed and sensitivity required for hearing,” stated Dr. Tobón.
Key Findings:
- Neurotransmitter release occurred at distinct sites where a glutamate-filled vesicle was coupled to an adjacent calcium channel. On average, only 1-3 channels contributed calcium to trigger release from each site.
- Binding of ~4 calcium ions was necessary to trigger the fast release of neurotransmitters from the vesicle. This likely reflects binding to the multi-domain calcium sensor protein otoferlin.
- During physiological stimuli, the relationship between calcium influx and glutamate release was nearly linear. This enables the hair cell synapse to reliably encode timing and intensity of sounds.
Implications:
According to senior author Dr. Tobias Moser, “This work exemplifies research fostered by the Multiscale Bioimaging Cluster of Excellence, applying innovations in optical imaging and electrophysiology to uncover molecular underpinnings of hearing.”
By showing that calcium entry through few channels governs vesicle fusion, the results aid efforts to understand noise damage and drug ototoxicity targeted to these channels.
The authors suggest that communication defects at this first synapse in the auditory pathway likely contribute to common hearing disorders. “Better grasp of sound encoding at hair cell ribbon synapses will help guide treatments to protect and restore hearing,” stated Dr. Tobón.
Overall, the findings reveal the intricacies of neurotransmission at individual sensory synapses. The quantitative insights provide a conceptual and methodological framework for probing mechanisms underlying auditory function as well as dysfunction in disease.
Reference:
- Lina María Jaime Tobón et al. (2023) Ca 2+ regulation of glutamate release from inner hair cells of hearing mice. Proceedings of the National Academy of Sciences. DOI: 10.1073/pnas.2311539120