Our ears have an amazing ability to hear very faint sounds and distinguish different sound frequencies, even amidst background noise. This exceptional hearing sensitivity relies on a special process of sound amplification within the inner ear.
Now, scientists have gained important new insights into how this amplification process works at the molecular level.
Prestin’s “Molecular Elevator” Mechanism Revealed
Within the cochlea, the outer hair cells possess a distinctive ability to alter their cell length in response to the vibrations induced by sound entering the ear. This feature, termed electromotility, causes the cells to pump energy into the sound vibrations, actively amplifying quiet sounds in a process called cochlear amplification.
Electromotility relies on the activity of a protein called prestin that exists in high amounts within the membrane surrounding outer hair cells. When sound waves cause the cell’s membrane voltage to fluctuate up and down, prestin molecules rapidly change shape in sync with the voltage changes, making the cells contract and expand repetitively.
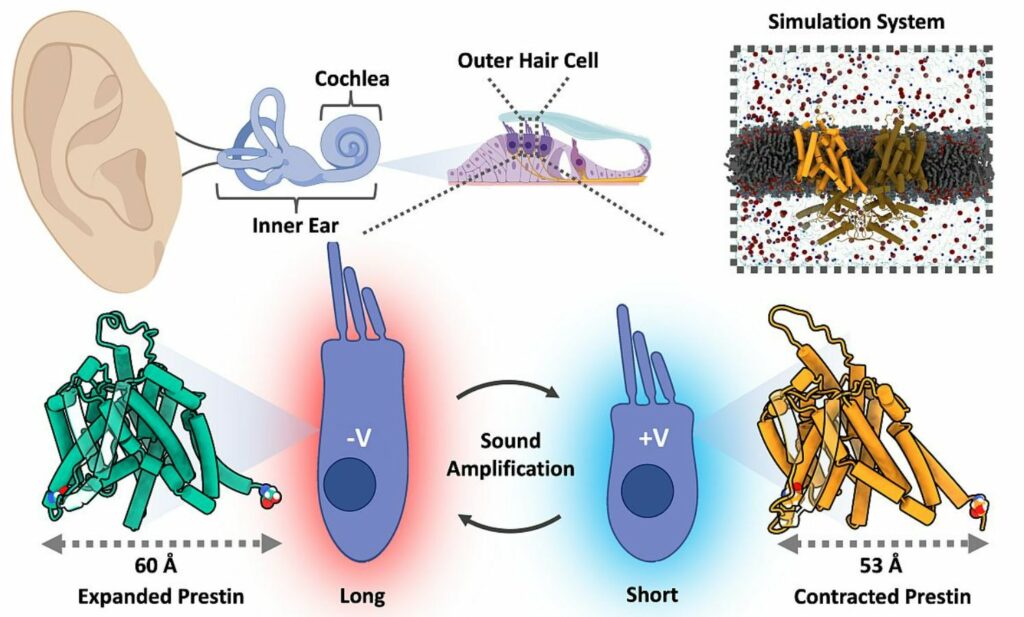
Prestin-mediated sound amplification. Outer hair cells, located in the Organ of Corti rapidly contract/expand to amplify the sound-signal sent to the brain; Prestin’s voltage dependent ‘Elevator-mechanism’ is responsible for expansion/contraction. Image credit: Kuwabara, M.F. et al. via Forschungszentrum Juelich
While previous studies identified important structural states of prestin, exactly how this shape-changing ability translates into cellular electromotility remained uncertain.
Confirming the Mechanism Through Experiments
Now, a team led by scientists from UConn Health Center used computer simulations and experimental techniques to map out the dynamic motions prestin molecules utilize to drive cellular shape changes underlying cochlear amplification. Their findings show that prestin employs a “molecular elevator” whereby a central transport unit slides up and down relative to an immobile scaffolding structure in response to voltage changes. This movement transduces voltage fluctuations into directional cell-length changes.
The studies found prestin alternates between compact and expanded conformations due to the transport unit moving downward or upward respectively relative to the scaffolding anchor. The expanded state increases the cell’s dimensions, while the compact state decreases dimensions, together enabling voltage changes to rhythmically extend and contract the cell.
Researchers confirmed the elevator-like motion experimentally by showing that constraining the sliding behavior using chemical crosslinks greatly hampered the cell’s ability to change shape. They further demonstrated the voltage-dependency of the shape changes by attaching fluorescent probes to report on structural movements in response to controlled voltage fluctuations.
Reference:
- Kuwabara, M.F., Haddad, B.G., Lenz-Schwab, D. et al. Elevator-like movements of prestin mediate outer hair cell electromotility. Nat Commun 14, 7145 (2023). https://doi.org/10.1038/s41467-023-42489-8