BOSTON, MASSACHUSSETTS — Researchers at Harvard Medical School (HMS) have made significant progress in developing a gene therapy for Usher syndrome type 1F, a rare genetic disorder characterized by congenital deafness and progressive vision loss.
The new approach, detailed in the Journal of Clinical Investigation, demonstrates restored hearing and balance in animal models and holds promise for improving vision in future treatments.
Usher syndrome type 1F is a debilitating condition caused by mutations in the PCDH15 gene. People with this syndrome are born deaf, lack a sense of balance, and gradually lose their vision over time. The research team, led by David Corey, the Bertarelli Professor of Translational Medical Science at HMS, has devised a novel gene therapy approach that could address both sensory deficits.
“Without a clinical trial in humans, we can’t know whether our first gene therapy restores normal function. This new strategy gives us a backup in case the first therapy doesn’t work. It might even turn out to be better than the first, once tested in patients.”
–David Corey, Ph.D.
A New Gene Delivery Method
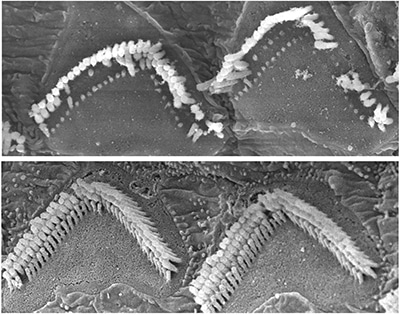
Mouse models of Usher syndrome type 1F show abnormal hair cell bundles in the inner ear (top). The dual-AAV therapy improved cell structure and function in these mice (bottom). Image credit: Maryna Ivanchenko, Harvard HMS
Gene therapy for Usher syndrome poses a unique challenge because the PCDH15 gene is too large to be carried by a single adeno-associated virus (AAV), the most commonly used and safest gene delivery vehicle. To address this, Corey’s team has developed a dual-AAV approach.
Instead of trimming the gene to fit within one AAV, as done in previous research, they split the full-length PCDH15 gene into two separate pieces, each loaded into an individual AAV. When delivered to target cells in the inner ear or eye, the two halves reassemble, enabling the cells to produce functional protocadherin-15 protein.
The results have been promising. In mouse models of Usher syndrome type 1F, the gene therapy successfully restored hearing and balance, indicating that the dual-AAV method effectively delivered the full-length gene. The improvement in hair cell structure and function in the inner ear was evident, as shown in electron microscope images that revealed significantly restored cell architecture. However, since the vision loss associated with Usher syndrome does not manifest in these specific mouse models, the team turned to alternative systems to evaluate the therapy’s effect on vision.
Vision Potential Demonstrated in Organoids and Primates
To test whether the gene therapy could impact vision, the researchers used human retinal organoids and nonhuman primate retinas. The dual-AAV therapy led to increased levels of protocadherin-15 in the light-sensing cells of these models, and the protein localized correctly within the cells, a crucial step for normal retinal function. While these findings do not yet confirm vision restoration, they suggest that the therapy has the potential to slow or prevent vision loss in people with Usher syndrome type 1F.
“These results are particularly exciting because, while cochlear implants can address hearing loss in human patients, there are currently no treatments for the vision dysfunction associated with Usher syndrome”
–Maryna V Ivanchenko, M.D., Ph.D., study author and HMS Instructor
The significance of this research lies not only in the demonstrated hearing restoration but also in the development of a possible strategy for vision preservation. The hope is that this dual-AAV therapy could provide a comprehensive treatment for Usher syndrome, addressing both auditory and visual deficits.
Collaboration and Future Outlook
The study represents a collaborative effort between the Corey Lab at HMS, the Institute of Molecular and Clinical Ophthalmology Basel, and the University of Basel in Switzerland. It highlights the importance of teamwork in advancing genetic therapies for rare and complex conditions.
Moving forward, the team acknowledges that more research is needed before clinical trials in humans can begin. The dual-AAV approach must be rigorously tested to ensure its safety and efficacy. Still, the researchers are optimistic about the potential of their work to change the standard of care for Usher syndrome type 1F.
“The landmark change in criteria is a crucial step in addressing the underutilization of cochlear implants and ensuring that more individuals with hearing loss can benefit from these life-changing devices,” added Kevin Brown, M.D., Ph.D., Chief of the Division of Otology and Neurotology at the University of North Carolina-Chapel Hill and a study investigator.
The work was supported by grants from the National Institutes of Health, the Bertarelli Foundation, the Seamans Family, and the Swiss National Science Foundation. Continued support from the Usher 1F Collaborative has been instrumental in advancing this research, underscoring the impact of dedicated advocacy and funding in driving scientific progress.
As the research team prepares for the next steps, the hope remains that their innovative approaches will lead to transformative treatments for individuals with Usher syndrome, offering the possibility of restored hearing and preserved vision in the future.
Reference:
Ivanchenko, M., Hathaway, D. M., Mulhall, E. M., Booth, K. T., Wang, M., Peters, C. W., … & Corey, D. (2024). Dual-AAV Vector Delivery of PCDH15 for Usher Syndrome Type 1F: Hearing and Vision Preservation. Journal of Clinical Investigation.
*A video discussing the Corey Lab’s gene therapy strategies can be watched below:






