While there continues to be a growing number of companies developing drugs to treat inner ear disorders, the inner ear remains a particularly challenging organ for targeted drug delivery. Now, however, a novel device to deliver drugs to the inner ear will undergo preclinical testing in an industry consortium.
With funding from the National Institute of Deafness and Other Communication Disorders (NIDCD) and in collaboration with clinicians and researchers at Massachussetts Eye and Ear, Draper developed the novel drug delivery device.
“Today’s treatment of inner ear diseases is hampered by the shortcomings of available drug delivery technology. For diseases of the inner ear, clinical research and treatment have been held back by the need for a safe, direct and effective intracochlear drug delivery method. We address this challenge with Draper’s device—the first implantable and programmable micropump that provides targeted, controllable and extended drug delivery. With this consortium we can advance development and optimize the intracochlear drug delivery (ICDD) as well as accelerate testing and delivery of hearing loss drug candidate compounds and combination therapies on behalf of researchers who invest in the consortium evaluation program.” –Jeff Borenstein, Lead Scientist for Drug Delivery at Draper
Established in Boston, the CILcare-CBSET-Draper consortium will work to to assess the ICDD device using CILcare’s hearing loss preclinical models, and the operational support of CBSET’s laboratories.
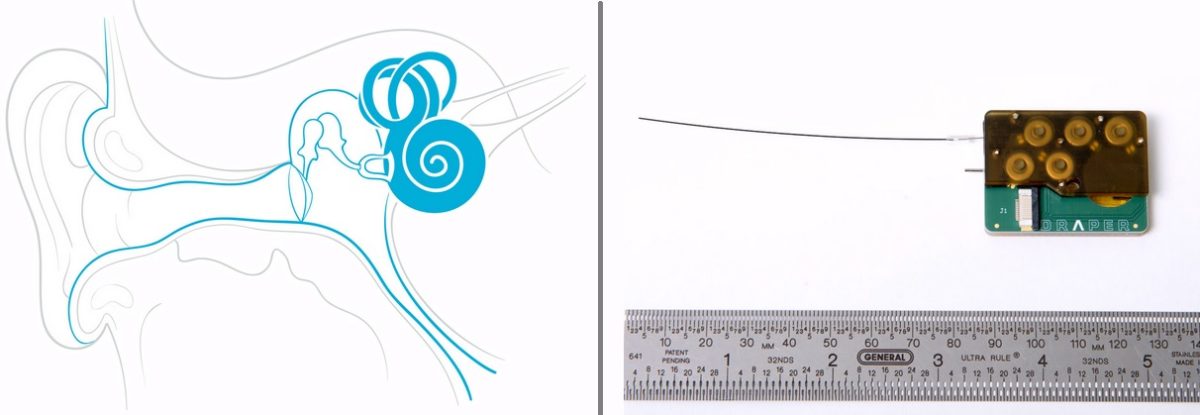
Draper’s new intracochlear drug delivery device shown on right.
Drug Treatment of Hearing Loss and Inner Ear Disorders
According to the announcement, the trio of companies will invite other biopharmaceutical and pharmaceutical companies involved in ear disorders into the consortium.
“Hearing loss disorders are a global phenomenon, but there is currently no drug to treat the condition. Our consortium aims to provide the partnered companies with proof-of-concept data on the efficacy of delivering their compounds using Draper’s ICDD in animal models, an indispensable step before going into human clinical trials.” –Célia Belline, CEO of CILcare
The program will be led and managed by CILcare in collaboration with CBSET, a non-profit research institute that specializes in the advancement of novel therapies.