IOWA CITY, IOWA — iotaMotion Inc., a leader in developing advanced robotic-assisted systems for cochlear implant surgery, announced today the company has surpassed an important milestone of 125 clinical use cases with the iotaSOFT® Insertion System.
Gaining authorization by FDA via a De Novo pathway in Q4 2021, iotaMotion began the Limited Market Release (LMR) of iotaSOFT in Q3 of 2022 at select centers across the country. iotaMotion has leveraged this early release to garner initial feedback from surgeon partners, expand training, and build up internal support infrastructure. With the LMR coming to a close, iotaMotion will continue to partner with these initial centers and expand into additional hospitals and ASC’s in 2023.
The University of Utah Hospitals, which recently acquired the system, completed the 125th use case with iotaSOFT during a case with Richard Gurgel, MD, MSCI. Dr. Gurgel, Professor of Otolaryngology at University of Utah, commented “The use of iotaSOFT provides exceptional control of the speed and resulting force during the insertion of a cochlear implant electrode. We know the cochlea is a delicate structure, and we are excited about adding iotaSOFT as a surgical tool to deliver the best possible care for our patients.”
iotaSOFT Insertion System, recently awarded as the Innovation of the Year by Hearing Health & Technology Matters, is the world’s first FDA-authorized robotic-assisted insertion technology intended to aid surgeons in the placement of the cochlear implant electrode array by controlling the speed of insertion.
Consisting of a thumb-sized bone mounted drive unit and a reusable console with foot pedal control, the system stabilizes the electrode throughout the insertion and controls the variability in forces seen within the cochlea. The system was designed for a seamless integration in the OR enabling the surgeon to insert the electrode array into the cochlea at a slower and more consistent rate than the human hand.
Dr. Oliver Adunka, Division Director of Otology, Neurotology & Cranial Base Surgery at The Ohio State University Medical Center, a key collaborator in the Limited Market Release, provided the following perspective on the iotaSOFT Insertion System:
“Giving up natural hearing while getting a cochlear implant is a main reason many candidates choose not to proceed with surgery. Robotic electrode insertions, as provided through the iotaMotion system, are an essential step in reducing the risk for device related hearing loss and are the future of cochlear implantation.”
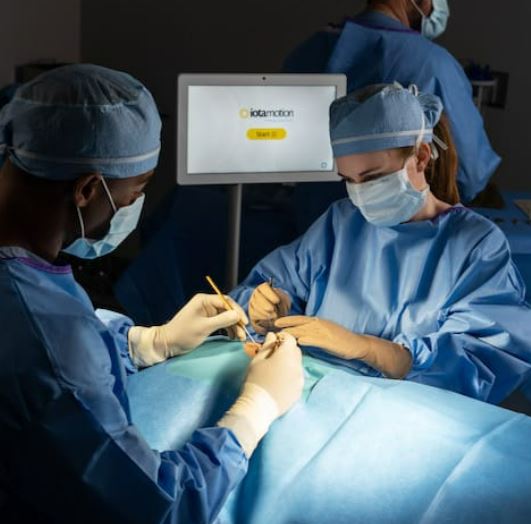
iotaMotion plans to expand to more centers in 2023
iotaSOFT will expand to many top cochlear implant centers throughout the country as part of an expanded, controlled market release. Throughout 2023 and beyond, iotaSOFT will be used to advance cochlear implant surgery forward and increase access and awareness of cochlear implants.
Mike Lobinsky, CEO of iotaMotion, commented “We are excited to see the growing adoption of iotaSOFT. Over the next year, we expect increased awareness and use of our iotaSOFT system across the country with substantially more centers and patients benefitting from this enabling technology.”
To learn more, visit the company website here.
About iotaMotion Inc
A privately held Iowa based company, iotaMotion is developing surgical robotic assistive technologies with the goal of advancing cochlear implant surgery beyond human capability. The company’s solutions aim to standardize cochlear implant electrode array insertion, and to provide unprecedented control in surgical care settings with the goal of expanding access to cochlear implant interventions for both surgeons and patients.
Source: iotaMotion






