LAS VEGAS, NEVADA — Nuheara, the smart-hearing company, today announced the commercial availability of its HP Hearing PRO. Consumers can now pre-order the HP Hearing PRO self-fitting, air-conduction, OTC hearing aid at www.HPhearingPRO.com and soon be able to purchase the product from any retailer carrying the product including Best Buy and Crutchfield.
Nuheara’s HP Hearing PRO is the first product cleared through the FDA’s 510(k) process for both 874.3325 self-fitting and the 800.30 OTC hearing aid classifications under a new product classification code “QUH”. HP Hearing PRO is the first FDA cleared self-fitting OTC hearing aid with Hybrid Active Noise Cancellation. The innovative Ear ID™ software-in-medical-device and earbud form factor sets the HP Hearing PRO apart from other devices on the market1.
 The HP Hearing PRO self-fitting OTC hearing aids are designed to look more like an earbud rather than a traditional hearing aid. The HP Hearing PRO self-fitting OTC hearing aid brings together “superb medical-grade hearing aid technology with the highly desired features of wireless earbuds into a multifunctional device”.
The HP Hearing PRO self-fitting OTC hearing aids are designed to look more like an earbud rather than a traditional hearing aid. The HP Hearing PRO self-fitting OTC hearing aid brings together “superb medical-grade hearing aid technology with the highly desired features of wireless earbuds into a multifunctional device”.
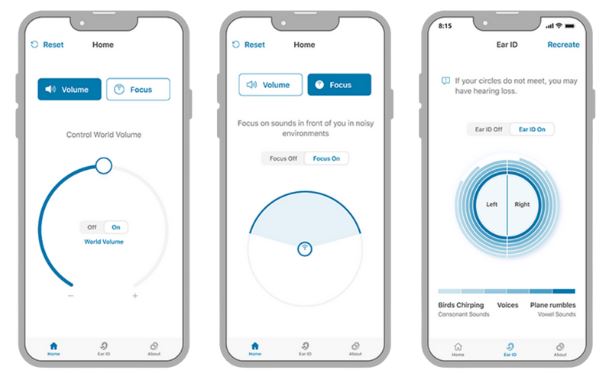
According to the company announcement, “users can enjoy clinically-proven natural sound quality2. The HP Hearing PRO provides a lifestyle-friendly hearing aid experience with support for situational use and clinically-proven 30% improved hearing using the Focus directionality feature, adjusted to the user’s preferences with a tap of the HP Hearing app.”
HP Hearing PRO OTC Hearing Aids: Availability and Pricing
Orders can now be placed from retailers and ecommerce partners throughout the US. The recommended retail price for a pair of HP Hearing PRO hearing aids along with an on-the-go charging case is US$699.00. Product is expected to ship to customers before the end of calendar Q1 2023.
Built to medical-device standards and meeting all FDA requirements for safety and efficacy, the HP Hearing PRO features Ear ID™ self-fitting software and technology powered by Nuheara. The HP Hearing PRO self-fitting OTC hearing aid has been “clinically proven to be substantially equivalent to a professionally fit hearing aid”.3 The proprietary Ear ID software tests the wearer’s individual hearing thresholds from low frequency to high frequency in each ear, then automatically programs the HP Hearing PRO hearing aids for each ear. This ability for the consumer to self-fit through the HP Hearing application, for iOS and Android platform mobile devices, allows a quick and easy acclimation experience for the consumer. The entire experience takes about 10-15 minutes from unboxing through customization of the user’s personalized profile to accommodate their perceived mild-to-moderate hearing loss.
The HP Hearing app is available for iOS and Android
In addition to the HP Hearing PRO’s capabilities as an OTC hearing aid, this innovative product streams media and phone calls via Bluetooth®. The Active Noise Cancellation minimizes background noise for an immersive sound experience during phone calls or when streaming music.
Additional features include:
- HP Hearing app for iOS and Android
- Rechargeable built-in Li-ion batteries
- Medical-grade USB-C charge case
- Battery life is up to 8 hours hearing aid processing per full charge per hearing aid
- Clinically-proven natural sound quality4
The HP Hearing PRO has been clinically validated5 through research completed by the National Acoustic Laboratories to provide a 30% speech understanding improvement in the presence of noise using the directional microphone feature called Focus. This is a huge benefit for consumers in restaurants or social situations where background noise makes understanding speech difficult. Now, at the touch of the HP Hearing app, a wearer can switch the directional microphone settings to Focus on sounds towards their front, quickly allowing for immediate improvement in speech understanding.
 The HP Hearing PRO is a CES® 2023 Innovation Awards Honoree. Further information can be found at www.HPhearingPRO.com. The HP Hearing PRO will be on display at CES 2023, in the Las Vegas Convention Center North Hall Booth #8763.
The HP Hearing PRO is a CES® 2023 Innovation Awards Honoree. Further information can be found at www.HPhearingPRO.com. The HP Hearing PRO will be on display at CES 2023, in the Las Vegas Convention Center North Hall Booth #8763.
Learn more about the HP Hearing PRO powered by Nuheara in the CES Tech Talk podcast here: https://www.ces.tech/Events-Programs/CES-Tech-Talk-Podcast/Season-6/This-Earbuds-for-You-Hear-Better-Affordably.aspx
“Since establishing Nuheara in 2015, we have been driven by our ambitious vision that our affordable and leading-edge hearing devices could help to solve accessibility challenges for consumers. We are excited for the millions of Americans who are about to begin their hearing health journey now that the US’ FDA has made hearing aids available in an affordable over-the-counter option. Not surprisingly, the response from retailers and consumers alike has been strong.”
–Co-founder and Managing Director of Nuheara, Justin Miller
“Our team has worked hard to develop a safe, effective and affordable over-the-counter hearing solution, in the HP Hearing PRO. Thirty-eight million Americans have been waiting years to access an affordable option for their mild-to-moderate hearing loss needs – and now it is here,” said CEO of Nuheara John R. Luna.
“In the years since we first launched the IQbuds, thousands of Americans have come to Nuheara for the first product to begin their hearing health journey. Now that the US’ FDA has deemed the HP Hearing PRO substantially equivalent to an audiologist fit hearing aid, we’re ready to serve millions more. The powerful combination of Nuheara’s technology and experience along with HP’s brand promise, should give consumers confidence in their purchase of the HP Hearing PRO in this new OTC hearing aid category.”
ABOUT NUHEARA
Nuheara is a global leader in smart hearing technology which change people’s lives by enhancing the power to hear. As a global pioneer in Hearable products, Nuheara developed proprietary, multi-functional, personalised intelligent hearing devices that augments a person’s hearing. Nuheara is headquartered in Perth, Australia and was the first consumer wearables technology company to be listed on the Australian Securities Exchange (ASX).
In 2016, the Company released its revolutionary wireless earbuds, IQbuds, which allow consumers to augment their hearing according to their personal hearing preferences and connect hands free with their voice-enabled smart devices. In 2020 Nuheara released its third-generation hearable the IQbuds² MAX. In 2021, Nuheara transformed its operations to include medical device manufacturing for its hearing aid products to meet the global rise in mild-to-moderate hearing loss. Nuheara products are now sold Direct to Consumer (DTC) and in major consumer electronics retailers, professional hearing clinics, pharmacies, and speciality retailers around the world. In 2022, Nuheara’s HP Hearing PRO was the first product cleared through the FDA’s 510(k) process for both 874.3325 self-fitting and the 800.30 OTC hearing aid classifications under a new product classification code “QUH”.
The Company’s mission is to transform the way people hear by creating smart hearing solutions that are both accessible and affordable. For further information, please visit https://www.nuheara.com/.
1 510(k) Premarket Notification, US FDA: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K221064
2 Clinical Investigation Report: Validation of Nuheara Self-Fit Hearing Aid, Version 4,31st January 2022 by National Acoustic Laboratories, Australia
3 Ibid.
4 Clinical Investigation Report: Validation of Nuheara Self-Fit Hearing Aid, Version 4,31st January 2022 by National Acoustic Laboratories, Australia
5 Ibid.






