Oticon Medical has announced the U.S. Food and Drug Administration (FDA) clearance and CE mark approval for its first active transcutaneous bone conduction hearing system, the Sentio System. This new system offers the established benefits of the Ponto™ System and introduces a transcutaneous option.
The Sentio System consists of the Sentio 1 Mini, an external sound processor, and the Sentio Ti Implant, placed under the skin. It is currently the smallest available transcutaneous system.
Unlike percutaneous bone-anchored hearing systems such as Ponto, which use skin-protruding abutments, the Sentio System maintains intact skin. This design provides an alternative for patients, supporting Oticon Medical’s commitment to “freedom of choice.”
Oticon Medical Sentio System
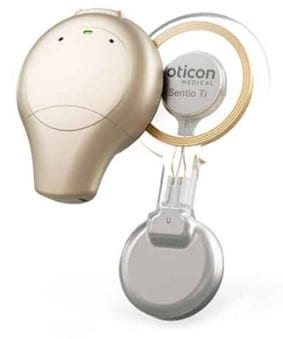 With the Sentio System, Oticon Medical offers a comprehensive portfolio of bone-anchored hearing systems to meet diverse clinical needs and global reimbursement schemes. The Sentio 1 Mini emphasizes user comfort and ease of use, featuring a slim profile that is 26% lighter than alternatives. It aims to deliver a 360-degree sound experience and offers the widest bandwidth at 9.5kHz.
With the Sentio System, Oticon Medical offers a comprehensive portfolio of bone-anchored hearing systems to meet diverse clinical needs and global reimbursement schemes. The Sentio 1 Mini emphasizes user comfort and ease of use, featuring a slim profile that is 26% lighter than alternatives. It aims to deliver a 360-degree sound experience and offers the widest bandwidth at 9.5kHz.
The Sentio Ti Implant is designed to support patients with progressive hearing loss without requiring additional surgeries. It is verified for a higher maximum force output to accommodate more powerful sound processors, ensuring reliability for patients. From a surgical perspective, the implant offers flexibility and a straightforward procedure.
The Sentio System is indicated for candidates aged 12 and older diagnosed with conductive hearing loss, mixed hearing loss, or single-sided deafness. This launch marks a significant milestone for Oticon Medical in delivering innovative hearing solutions.
For more information, visit www.oticonmedical.com.
**Not all products are available in all markets. Product availability and indications are subject to regulatory approval and may vary depending on the market.
About Oticon Medical
Oticon Medical is a global leader in implantable hearing solutions, dedicated to enhancing the power of sound for people at every stage of life. For over a decade, the company has made bone-anchored hearing systems more accessible, simplifying treatment for physicians, audiologists, and patients.
Oticon Medical believes in offering patients and hearing care professionals the best possible solutions at any point along the patient journey, encapsulated in their principle of “Freedom of Choice.” This commitment ensures that their solutions are designed to be compatible whenever possible, offering lifelong support for users.
Working collaboratively with professionals, Oticon Medical creates solutions that address users’ needs, aiming to enhance quality of life and help people live fully now and in the future. Because they understand the profound impact of sound.
References
1 Sentio implant and sound processor physical features and comparison to other devices (Doc-00123204)
2 275144en Product Information Sentio 1 Mini
Source: Oticon Medical






