DURHAM, NORTH CAROLINA – MED-EL USA has announced that the U.S. Food and Drug Administration (FDA) has approved the SONNET 3 audio processor for MED-EL cochlear implants. The company’s lightest and smallest behind-the-ear audio processor, SONNET 3 features integrated wireless direct streaming capabilities and a design optimized for bilateral use.
Features and Technology
SONNET 3 incorporates ASM 3.0 technology, which automatically adjusts processor settings based on the user’s environment. This includes reducing background noise and utilizing dual microphones to enhance speech focus during conversations.
The processor supports binaural streaming, allowing bilateral users to pair two SONNET 3 processors or a SONNET 3 with another MED-EL processor equipped with AudioStream. Users can stream music and phone calls directly from compatible Android and Apple devices, as well as smart TVs and digital media players, without requiring additional accessories.
Additional features include a Touch Key for quick program adjustments, a flexible earhook for comfort, and a waterproof IP68 rating for water-related activities.
“We are thrilled to introduce SONNET 3 to our current cochlear implant users and candidates in the United States. Our smallest and lightest behind-the-ear audio processor with built-in direct streaming is ready to fit seamlessly into everyday life. In addition to comfort and convenience, SONNET 3 offers the superior hearing performance that MED-EL is known for”
–John Sparacio, President and CEO of MED-EL USA
Design and Availability
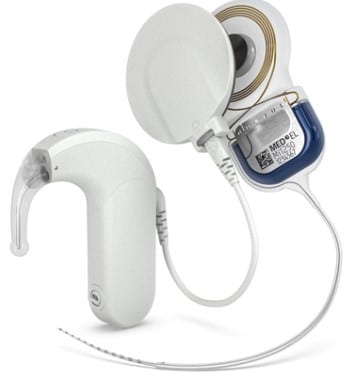 SONNET 3 is 2.0g lighter and over 18% shorter than its predecessor, SONNET 2 with AudioStream, offering a more compact and comfortable design. The device is expected to become available in early 2025.
SONNET 3 is 2.0g lighter and over 18% shorter than its predecessor, SONNET 2 with AudioStream, offering a more compact and comfortable design. The device is expected to become available in early 2025.
Through MED-EL USA’s TechSwap Program, patients who receive a MED-EL cochlear implant on or after September 1, 2024, will have the option to exchange one audio processor for the SONNET 3 when it becomes available.
About MED-EL
MED-EL Medical Electronics is a leader in implantable hearing solutions, committed to overcoming hearing loss as a barrier to communication and quality of life. The privately owned Austrian company was co-founded by Ingeborg and Erwin Hochmair, pioneers in cochlear implant research. Their work led to the development of the world’s first micro-electronic multi-channel cochlear implant, successfully implanted in 1977 and forming the foundation for modern cochlear implant technology.
Founded in 1990, MED-EL has grown to a global organization with more than 2,900 employees across 90 nations and 30 locations. The company provides a wide range of implantable and non-implantable hearing solutions, serving individuals in 137 countries. MED-EL’s portfolio includes cochlear and middle ear implants, electric acoustic stimulation systems, and both surgical and non-surgical bone conduction devices.
For more information, visit www.medel.com.
Source: MED-EL






