By: Diana Holan, MS
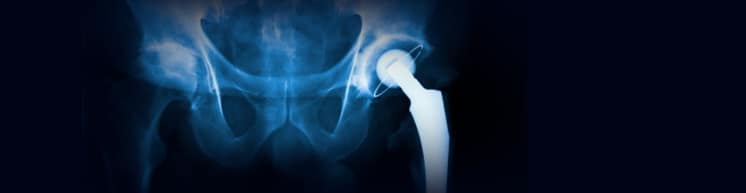
There are currently more than 250,000 hip replacements performed in the US annually. This procedure was relatively uncommon before the late 1990s, but with the development of titanium and cobalt/chromium metal-on-metal (MoM) bearing surfaces, sales rapidly increased and a highly competitive market was born. But new health concerns soon arose because the cobalt ions easily pass into the blood stream to the rest of the body (even into umbilical cords) and cause “metallosis” from the implants, known as “arthroprosthetic cobaltism.” Although the dangers of cobalt toxicity in humans have been known since the 1970s, these metal devices were developed outside the U.S. and slipped through the usually stringent premarket test requirements for human safety. According to the FDA, there were 16,800 reported implant problems from 2000-2010 and 14,000 implants were replaced. In March 2013, DePuy Orthopedics, Inc. (a Johnson & Johnson subsidiary) was ordered to pay more than $8.3 million in the first of almost 11,000 lawsuits involving their hip replacement product.
Originally designed in Birmingham, England, the MoM devices were touted to last significantly longer than previous ceramic materials. However, they soon proved to have very high surgical revision rates (a second hip replacement) due to tissue damage and localized pain cause by the cobalt/chromium flaking off into the surrounding tissues. Depending on data sources, overall implant failure rates should range from 1%-5%, while these metal products failure rates range from 12% – 40% in just a few years (based on full or partial revision results). Along with typical joint failure issues, there are additional post-op problems for women and smaller men because of reduced joint lubrication. Revisions of two Stryker products (another Johnson & Johnson subsidiary) are said to be the most painful because the stem must be chiseled out of the femur and away from the bony growth that develops from the original implant. The replacement stem is larger and longer and may require “banding” to hold it to the femur. “Even with the most skilled surgeons…post-operative complications, especially infections and fractures, are common.”
Cobalt poisoning has documented and serious side effects, including heart problems, reduced cognitive/memory function, DNA changes, optic nerve demyelination, neuropathy, renal failure, hypothyroidism, cancer, extreme fatigue, vertigo, tinnitus and sensorineural hearing loss. Published case reports demonstrate how arthroprosthetic cobaltism symptoms may masquerade as other serious health issues, and can occur within a few months and worsen over time if untreated.{{1}}[[1]]Tower S. Arthroprosthetic cobaltism: identification of the at-risk patient. Alaska Medicine 2010; 52: 28-32.[[1]] One Australian man had reduced energy, severe cognitive problems and pain in his limbs, but no hip pain or swelling. An Australian woman experienced CVA symptoms almost 4.5 years after implant, including imbalance and problems with auditory comprehension. Two Alaskan men and a Japanese woman also had toxicity symptoms, including hearing loss, although their pre-/post-audiograms were not included. In all these cases, serum cobalt levels were very high, so revisions were completed and symptoms either disappeared or improved with time. However, a Czech man was left with “…complete deafness (AD) … and 83% hearing impairment, (AS)”6, even after revision. Other patients still have not returned to a state of pre-surgery “normal”, but overall, there is limited data on the long term effects.
Metal-on-metal implants were initially distributed through a product category that required mechanical testing only outside the body. Later modifications were approved because they were deemed similar to those previous designs. This helped the manufacturers avoid the usual pre-market testing for human efficacy that includes expensive and long-term postoperative evaluation. According to internal company emails that were revealed in court and elsewhere, the higher failure rates and cobalt dangers were known by the largest manufacturer, DePuy, as early as 2005, but they waited until 2010 to recall their 93,000 implanted hip replacements. Four of their competitors–Wright Medical, Biomet, Zimmer, and Smith and Nephew–followed shortly with their own recalls. Also in 2010, Stryker Orthopedics (another Johnson & Johnson subsidiary) released a ceramic-on-polyethylene bearing surface product that was supposed to eliminate the source of the metallosis, but similar problems occurred due to “metal-on-metal articulation….at the neck-stem interface.” It was recalled in 2012. And an actual human trial of a Smith & Nephew large diameter MoM was ended after two years because “…20% (of the) patients…had raised metal ion concentrations in the blood.”
Regulatory agencies in several countries were slow to respond to medical and patient concerns, most likely due to commercial influence. In May 2011, the FDA ordered DePuy and 21 other MoM manufacturers to at least provide ongoing monitoring for toxicity. It reclassified all hip implants as “high risk” in January 2013 and established even more stringent rules for pre-surgery patient education and post-surgical patient monitoring. It also funded a study of existing implant registries to begin evaluating the data. Some medical and scientific personnel believe some MoM surgeries will prove successful when based on higher surgeon skill level and specific product design, so they are calling for a massive prospective outcome evaluation. But there is disagreement on what constitutes a normal and safe cobalt level, as well as what criteria should be used to recommend explanting the hips.
In the meantime, there are variables that will potentially have a negative economic impact. The financial cost of all those revision surgeries, follow up care and potential long term care will be factored into insurance costs, which will then increase rates. There are important public safety considerations for current and future implant patients who work in high functioning jobs, e.g.. pilots and medical professionals.{{2}}[[2]]Sotos JG & Tower SS. Systemic Disease after Hip Replacement: Aeromedical Implications of Arthroprosthetic Cobaltism. Aviation, Space and Environmental Medicine, 84. 2013. pp 242-2.[[2]] In this age of medical specialization it is yet one more related question to be explored in patient histories, which can increase appointment times and add to time and cost of referral tests. And certainly, it is a daunting, emotional task for the hundreds of thousands of people worldwide to choose between surgery and long term monitoring. The full picture of the metal-on-metal hip replacement may not be complete for decades.
A good friend has worn powerful binaural BTEs (post stapedectomy) for about 50 years. I have known him since 1998. He scheduled a follow up audiologic evaluation in March because, although his hearing aids were functioning well, they were not adequate. During the test, I noticed that he kept falling asleep – even when holding his arm up for pure tone responses. After the word lists were completed, I opened the booth door, walked two feet in and had to shake him several times to wake him again. I was worried because I had already noticed that he looked quite worn out. So he told me the story of his last hip implant in 2010 (it was his eighth joint replacement) and the subsequent symptoms that were sudden and unexplained: (1) shortness of breath, sudden lack of energy, plus continual pain at the hip site, which meant he could no longer golf, volunteer or continue as usual in the rest of his very active lifestyle; (2) and this fatigue, which meant he often fell asleep during the day, something he had never done before. He has also started developing macular degeneration in his right eye. He had some additional conductive loss, but his hearing does fluctuate.
Since our appointment, he had revision surgery April 8 (after an antibiotic regimen), but then he had post-op pain and infection that did not resolve, so he had to have another revision surgery on April 26. After spending time in the rehab hospital, he has just gone home. Reportedly, he is feeling much better overall, but is continuing on a six-week antibiotic treatment. His cobalt serum level was 10 times the normal rate pre-surgery. He will have that measured in the next few months and regularly monitored after that. He will also have follow up hearing and vision tests. He is one of the almost 11,000 people filing that lawsuit. Our office has added this question to our case history forms.
Diana Holan, MS, has been practicing audiology for over 20 years in Tucson and is committed to improving communication between patients and their families through the use of state-of-the-art hearing aid technology and various assistive techniques. She received a Bachelor’s of Science in Speech and Hearing and a Master’s of Science in Audiology from the University of Arizona








Interesting that you posted this. I had a patient about 9 months ago that had revision surgery on her hip due to this exact cobalt poisoning and the difference in her health was night and day. She was seen the previous year for vertigo and disequilibrium issues at our clinic and after addtional testing, it was ultimately determined that the source of her problem was this hip, which was then replaced and resulted in a tremendous improvement in her health.
Thanks for bringing attention to this issue!
Thank you for adding your insight!
I had my THR in 2008. Since last Easter I am suffering severe sinusitis. My cobalt levels have gone up 400% since last year. I have severely impaired hearing. Currently I am on steroid spray and am expecting to undergo a FESS and grommet operation. My concern is that both specialist in my case do not believe that my health problems are cobalt related! I also have no sense of smell whatsoever. I wish to find a doctor who will look into the toxicity I am suffering.
Hopefully if you bring in information to your Dr from what has been proven in others they will take a closer look to your particular situation. Hopefully you will get answers!
My had both hip replaced now I finding that my cobalt is increasing rapidly. What happened to rules and regulations the FDA frigged up and no cares.We should sue the shorts off them the GOVERMENT for allowing this.Were spending money on WAR and producing 99 cents hips.
My wife had the dePuy hip replacement several years ago and now has higher cobalt levels. Her doctor wants to operate again but she is 84 and her other hip is also bad. We just don’t know what to do because not operating will surely mean hog her levels of cobalt, yet the operation itself deeply concerns us because we don’t know whether her body can take it. We would appreciate any information we can get
I had hip replacement in mid 2009 and by early 2011 the pain in that hip was so bad that I had to have it taken out and replaced with a different type. My doctor had put in the metal on metal in 2009. Its just that I was 48 years old with a young son and because of the pain in the (mom) hip I was unable to enjoy so much that I wanted to but couldn’t do with my kid. With the last replacement ( so for OK) . Hope everyone finds comfort.
What kind of settlement are people gettting for the revision from finding out that their cobalt and chromium levels were too high?