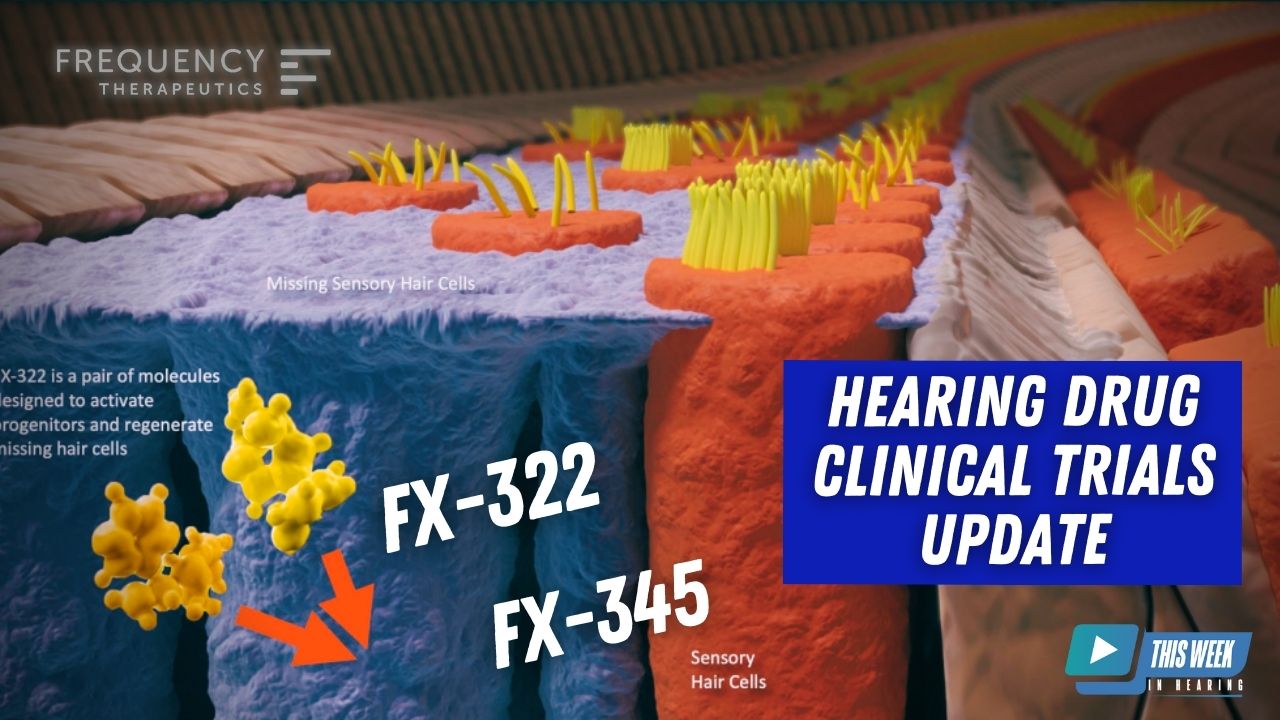
Nearly one year ago, Drs. Kevin Frank and Carl LeBel were on TWiH to discuss the exciting possibilities of regenerative medicine in hearing and the incredible progress, Frequency Therapeutics, has made on understanding the potential of applying it to clinical practice.
This week they joined Brian Taylor to update us on two clinical trials (FX-322 and FX-345) and how the results of these two trials could expand the scope of hearing restoration in the future.
Full Episode Transcript
Brian Taylor 0:10
Hello, and welcome to another edition of This Week in Hearing. I’m Brian Taylor. This week we have an update on regenerative medicine and hearing restoration with Kevin Franck and Carl LeBel of Frequency Therapeutics. viewers might recall that about a year ago, both Carl and Kevin, joined me to talk about the promise of hearing restoration. And they are here today to update us on some of their progress on clinical trials. I’d like to take the time now to welcome back to this week in hearing, Carl, and Kevin.
Kevin Franck 0:36
Thanks, Brian.
Carl LeBel 0:37
Thank you, Brian. Good to see you again.
Brian Taylor 0:39
Yeah, good to see you, too. I think a good place to start would be to kind of remind our viewers about what therap – what your company frequency Therapeutics is up to what you’re trying to accomplish in the hearing care market.
Carl LeBel 0:54
Yeah, I’d be happy to start and bring Kevin into the discussion. So I think the our major emphasis of focus has been on this Phase 2B study that we’ve been running in patients that have acquired sensory neural hearing loss. And this is with our lead candidate, I’m hearing restoration candidate FX 322. We announced not too long ago, by approximately October of this year, that we completed enrollment in this large study. So maybe we’ll just take a little bit of time to talk about the scale of the study. It’s the largest study that we’re aware of double blind, placebo controlled study that’s been done in this space before, looking to see if there’s a potential therapeutic, that can help restore some hearing function in folks that have lost their hearing due to a couple of different etiologies. Those two etiologies that we’ve been studying are patients that either have noise induced hearing loss, or they have experienced idiopathic sudden sensorineural loss, which is permanent. So we’re in the more chronic phase or permanent phase of hearing loss for those folks. So we designed a study where we’re comparing Fx-322 to placebo. And this study was designed to recruit 124 subjects equally balanced between those two groups, we ended up recruiting 142 subjects into the study. Now, this is after approximately 11,000 people expressed an interest in participating in the study. So a lot of folks were interested in seeing if they could potentially be candidates, because our criteria to participate in the study are pretty strict. And I’ll let Kevin speak to some of those, we ended up with 142. And so we’re pleased that we were able to recruit through some pretty challenging times, like a pandemic, as well as some natural disasters that we all deal with wherever we live. And all that is really a result of the dedication of our clinical trial sites, the audiologists that work on our on our study, importantly, the patients that have volunteered to participate in the study. So with recruitment completed, in a roughly October of this year, we are now disclosing that we would expect the results of the study will be available in the second half of the first quarter of next year. Okay, that’s when we’ll be able to disclose what’s happened and the primary endpoint to determine efficacy for the study. So so we are no longer discussing a signal or a hearing signal. Now we’re going to definitively test for efficacy to hopefully be able to show the effects three to two is effective in treating, acquired sensorineural hearing loss. So as I said, those results will be available second half of the first quarter, the primary endpoint is a measure of speech perception. And that is that is a test that’s standardized in the industry, Kevin can talk further to it. We have alignment with FDA that that is the primary endpoint. And that’s how the study was sized with that many subjects to be able to detect that difference. So all of the learnings that we have been a been collecting in the last five or so years, we’ve incorporated into this design, and it’s a really unique design for this field to be testing patients that have hearing loss. And maybe I’ll ask Kevin to share what some of the design features are for the study. Yeah, happy
Kevin Franck 4:49
to and Brian, thanks for the opportunity to chat. Then before I dive into those design features, it’s just such an exciting time for the company. You know, we’ve been thinking about hari cell regeneration since grad school, right? It was always a mystery why animals could do it, some animals could do it, but us mammals couldn’t. And here we are, right, kind of on the eve of the state of readout, when we’ve got a study designed to really show the effects of this drug. So it’s a very exciting time here at Frequency. And there’s some really amazing things coming down the pipeline, and, and what a thrill to be a part of it. So, as Carl said, you know, this, we’ve had five human clinical trials so far, and we’ve learned from every one of them, you know, we’ve learned that the drug is safe, you know, and over 200 people, no drug related serious adverse events, we learned who to study, right. So some of our studies replicated results in those populations, Carl mentioned, these people with, you know, the permanent, sudden idiopathic hearing loss and noise induced hearing loss. And then the third thing is how to study them. Because placebo controlled clinical trial like this really hasn’t been run to our knowledge. And so in many ways, we had to break new ground. And when you use measures in speech perception, where the effort that a patient brings to the study, really matters, like we all know, is audiologists you can have some days where people just aren’t as, as into the test if you’re not properly managing them. And so, so much of what went into the study is ensuring we got patients who could have consistent attention. So I will walk you through the different ways we did that. So one way is before any patient would even be considered having the drug, they would be scheduled for three different visits, and three different visits that really establish not only their baseline, but the stability of that baseline. And so by having three visits and looking at them over time, you could see if in this whole time, no one knows, but asking the IRB, what it takes to get into the trial. So patients are coming in with a big battery of tests, and not really being sure no one’s really sure, which is the test that’s most important. So So during those three visits, we can see not only do they meet the entry criteria, because we had an extra criteria based on both pure tone average and speech perception scores. But we can also see did those any of those scores change over those three visits for whatever reason. And if they did change, those patients were excluded from the study, because there was nothing going on with them, that should cause a change. So we just don’t want anyone that exhibits that variability. Now, lastly, we recorded everything. So we had a camera on the audiometer. And we had a microphone on the subject. So we could really ensure that the test parameters, were just as we wanted them to ensure that they were always at the same level as specified early in the trial, we could make sure that the instructions were given consistently, for every case, encouraging guessing, and that if anything was going on in the booth, you know, where the patient seemed like they were getting sleepy that there’ll be a break, you know, just just as good hygiene of how you do an excellent speech perception test. And across all of our different sites, and all our different audiologists, we really need to ensure that that experience is very similar, that we didn’t do the quality control ourselves, we hired an external firm. So there’s, so there’s some distance between that work and us. But I think those features, right, so the multiple baseline measures, the ability to disqualify subjects who had any instability before getting the drug, the recordings to ensure that, you know, these these tests went just as we should. And the fact that no one knew the entry criteria, each of those we think are features of this. This this experiment, there are 208 clinical trial, that gives us a lot of confidence in what the results are going to be.
Brian Taylor 8:50
So one question I have is, this is the the FX 322 clinical trial that you’re that you’re talking about right now, this is what we would call phase 2B of the study, right? Correct. Okay, how is that different from past FX 322 studies that you’ve done?
Carl LeBel 9:10
Yeah great question. So all of the single dose trials, we’ve conducted to date four single dose trials, those were generally considered phase one studies, phase 1 B, studies that mean in patients that were have hearing loss, we conducted a repeat injection study of FX three to two that was what we call the 2a larger sample, not statistically power, no primary endpoint other than safety at that point. And so now you move to Phase 2 B, that’s generally where you have a primary endpoint to demonstrate efficacy. You’re always studying for safety. And then you power the study statistically, so that you can detect a difference and that then is what launches you into phase three, assuming success. Okay, that
Brian Taylor 9:59
and that next question I have is about the new clinical trial FX 345. So I saw that was recently something published online about an announcement about FX 345. So can you tell us about this new trial, and how it differs from FX 322?
Carl LeBel 10:18
Yeah, we’re really excited to be able to now take a second generation program into the clinic, all of the learnings that Kevin and I have been discussing from the clinical program on ethics through to all those design elements and understanding patient population and the testing measures and trying to standardize everything to try to reduce variability. All of that has been applied now to the FX 345 program. What makes us excited about 345 is the the drugs that comprise FX 322, it’s a combination of two drugs. One of those is a drug that targets what’s called glycogen synthase kinase or GSK. that drug is an inhibitor of that target. So we have exchanged the molecule in FX 322, for a more potent version, and that more potent version is now in FX 345. Because one of those drugs is more potent, it allows us we believe, to achieve therapeutic levels in a broader region of the cochlea, they go after the same targets. But we’re interested in if we can get more drug there and more drug throughout the cochlea, might we be able to potentially treat a broader patient population that has acquired sensorineural hearing loss? Or could the effects that we see in the existing populations have a greater magnitude of change. And those are possibilities that we’re interested in this first study, this 1B study, again, we’re doing safety in patients for the first time, we need to understand what the safety profile is. It’s a slightly broader population of patients that have acquired sensorineural hearing loss. But in general, most of the measurements and the requirements to get into the study are very consistent with what Kevin and I described on the on the 208, study the 2 B study for FX 322 that’s that’s ongoing. So our intent is to get that study to a place where we can have a readout and understand safety. And whether there are any changes in any of the audiological measures that we incorporate that would occur in the second half of 2023.
Brian Taylor 12:40
Kevin, I have a question for you you’re an audiologist tell the audiologist that are watching this, how these two studies or how these two applications of the drug maybe how they might benefit patients? Well,
Kevin Franck 12:59
so the what we’re seeing is a change in speech perception. Right. And we all know is speech perception as an audiologist when you hear a number of words what you can repeat back. And that’s what patients are asking for. They’re asking for that clarity. They’re saying, I hear you, but I don’t understand you. This is getting at the I understand you. And it’s doing so without changing audibility, right? So we’re doing the same loudness, before they get the drug as the loudness after they get the drug. And that’s the change we’re measuring. So for a given loudness, they’re able to hear more words. And you know, think of this as you know, watching TV with more pixels on the screen or whatever analogy you want, but this is getting at resolution clarity. And audiology does a great job of providing audibility, you know be that through a hearing aid or a cochlear implant, but we’re working on the cochlea itself to enhance the ability to perceive speech, which is exactly what patients are asking for. So that’s, that’s exciting, right speech, perception is always we’ve always done well with audibility and the perception that comes with that. But to have a drug that impacts perception without audibility is pretty exciting.
Brian Taylor 14:09
Right. I mean, I think you mentioned this when we had you on earlier this year. It sounds like there could be a future where hearing aids or cochlear implants provide audibility, and then a drug like yours provides clarity. Do I have that right?
Kevin Franck 14:25
I think more in the case of a hearing aid to be sure. Right. So you know, when you if the problem is you can’t hear anything, then the hearing aid will let you detect the sounds. And then with an increase in the resolution, things will sound better. So I think an audiologist could who knows have injections being done at the audiology clinic or they could send their patients to a place that does the injections. I think for a cochlear implant, I think it depends on whether you’re dealing with electro acoustic or where you are in the cochlea for that. But it is exciting because we see this as being quite integrated into audiologist practices.
Brian Taylor 15:03
That’s exciting. Anyway, before I let you go, any other, you know, like any other thoughts about where the profession is heading with respect to regenerative medicine or what your company might be up to over the next year. So, you know, please share what you can,
Kevin Franck 15:21
I think what we’re getting at is, you know how you can have a, imagine three patients one with a conductive hearing loss, and two with sensorineural hearing loss. As much as imagine they all have the same air conduction thresholds, you know, that that person with conductive hearing loss, if you can provide audibility, they perceive really, really well, because they’ve got a normal cochlea. And then two people with the same audiogram with sensorineural hearing loss can have really different speech perception abilities. And that difference really gets at what’s likely that underlying disability in the cochlea that we’re after. So whenever we think of patients whose speech perception is less than you’d expect it to be based on our ability, these are the people we’re most interested in studying and improving and in for us, that’s we’ve never been able to influence this right, beyond better microphones or, you know, the, you know, trying to make the signal cleaner. But to be able to get out that mystery that we’ve always thought of as audiologists is, why is it that this patient can’t perceive or the other patient can? And I think that’s, that’s right. That’s the action area of what we’re after.
Carl LeBel 16:25
Brian, I’ll just add, one of the things we haven’t talked about, that’s in the 208 study, we’ve been spending a lot of time for the last few years working on a new measure, it’s called a patient reported outcome measure. It’s an it’s a new measurement instrument. It’s a it’s a, it’s a questionnaire, and we call it radial, we’ve branded it radial, and it is really getting at the question of how does your hearing loss affect your daily activities? You think about all the things that you go through in a given day, if you have hearing loss? How are those affected? If you’re participating in a study with FX 322, and you are able to show improvements in speech perception? What else has changed in your life? Okay, in the beauty of that instrument is it’s actually designed by patients the requirements today, to create a new measure and a patient reported outcome measure. They always have to be designed by patients that have the condition. It’s not us sitting in our offices thinking up questions. So we’re really excited about seeing what the results are of that instrument along with hoping that we’re able to demonstrate changes and improvements in speech perception as well.
Brian Taylor 17:42
That sounds like a topic for another reason to have you on our broadcast. You said the outcome measures the acronym is radial, radial, correct. Yeah, it’s definitely something we’d want to talk about down the road somewhere. We’re always looking for. I’ll speak for myself as a clinician, it’s always good to find you know, better ways to measure and gauge outcome. So look forward to hearing more about that. Any other final thoughts? Carl or Kevin? Before we sign off?
Kevin Franck 18:11
Just grateful for your platform. It’s a it’s a great way to stay engaged and so glad that you had us on.
Brian Taylor 18:17
Yeah, no, thanks for taking the time.
Carl LeBel 18:20
thanks having us back and I look forward to sharing the results with you again real soon.
Brian Taylor 18:26
Kevin Franck Carl LeBel of frequency therapeutics. Thanks for coming on and talking about your clinical trials.
Carl LeBel 18:32
Our pleasure
Be sure to subscribe to the TWIH YouTube channel for the latest episodes each week, and follow This Week in Hearing on LinkedIn and Twitter.
Prefer to listen on the go? Tune into the TWIH Podcast on your favorite podcast streaming service, including Apple, Spotify, Google and more.
About the Panel
 Carl LeBel, PhD, has been the Chief Development Officer at Frequency since 2018. He started LeBel Consulting LLC after serving as the Chief Scientific Officer at Otonomy, Inc., from 2009 to 2016. Before joining Otonomy, he was President and CEO of Akesis Pharmaceuticals, Inc., a virtual metabolic disorders company, and prior to that spent more than 14 years at Amgen as an executive director in a variety of R&D management positions. Carl also worked as a research scientist at Alkermes Inc. and started his career as a consultant at Arthur D. Little, a management and technology consulting firm where he worked across several industries.
Carl LeBel, PhD, has been the Chief Development Officer at Frequency since 2018. He started LeBel Consulting LLC after serving as the Chief Scientific Officer at Otonomy, Inc., from 2009 to 2016. Before joining Otonomy, he was President and CEO of Akesis Pharmaceuticals, Inc., a virtual metabolic disorders company, and prior to that spent more than 14 years at Amgen as an executive director in a variety of R&D management positions. Carl also worked as a research scientist at Alkermes Inc. and started his career as a consultant at Arthur D. Little, a management and technology consulting firm where he worked across several industries.
Carl is a scientific fellow of the American Academy of Otolaryngology, and a full member of both the American Association for the Advancement of Science and the Society of Toxicology. He received his B.S. in Chemistry from the University of Detroit and his Ph.D. in Biomedical Sciences/Toxicology from Northeastern University. He was a NIEHS post-doctoral fellow in Molecular Neurotoxicology at the University of California Irvine. Carl is also a co-inventor on numerous patents in the field of drug delivery for otology-related disorders.
 Kevin Franck, PhD, is the SVP, Strategic Marketing and New Product Planning at Frequency Therapeutics. Kevin leads pre-commercial strategy and launch planning for the company’s clinical pipeline. He brings extensive experience in developing and commercializing products for people with hearing loss to Frequency.
Kevin Franck, PhD, is the SVP, Strategic Marketing and New Product Planning at Frequency Therapeutics. Kevin leads pre-commercial strategy and launch planning for the company’s clinical pipeline. He brings extensive experience in developing and commercializing products for people with hearing loss to Frequency.
A licensed audiologist, Dr. Franck joined Frequency from Massachusetts Eye and Ear, where he served as Director of Audiology and Harvard Medical School Faculty of the Department of Otolaryngology-Head and Neck Surgery. Prior to Mass Eye and Ear, he was Head of Marketing for Bose Hear, a division of Bose Corporation, where he led new product management and channel marketing of an emerging category of business focused on hearing loss. Dr. Franck co-founded Ear Machine, a startup funded by the National Institutes of Health before it was acquired by Bose in 2014. He has held senior roles at Cochlear Ltd., Artisan Healthcare Consulting and BiOM and worked as a clinician at the University of Michigan and the Children’s Hospital of Philadelphia.
Dr. Franck holds an MBA in Healthcare Management from the Wharton School of the University of Pennsylvania, a Ph.D. in hearing science from the University of Washington, an MSE in biomedical engineering from Johns Hopkins University and a B.A. in engineering from Dartmouth College. He is the incoming Board Chair of the Hearing Loss Association of America.
 Brian Taylor, AuD, is the senior director of audiology for Signia. He is also the editor of Audiology Practices, a quarterly journal of the Academy of Doctors of Audiology, editor-at-large for Hearing Health and Technology Matters and adjunct instructor at the University of Wisconsin.
Brian Taylor, AuD, is the senior director of audiology for Signia. He is also the editor of Audiology Practices, a quarterly journal of the Academy of Doctors of Audiology, editor-at-large for Hearing Health and Technology Matters and adjunct instructor at the University of Wisconsin.