In this week’s special episode, Dave Kemp catches up with Wade Colburn and Dr. Marlan Hansen of iotaMotion, a medtech startup spun out of the University of Iowa.
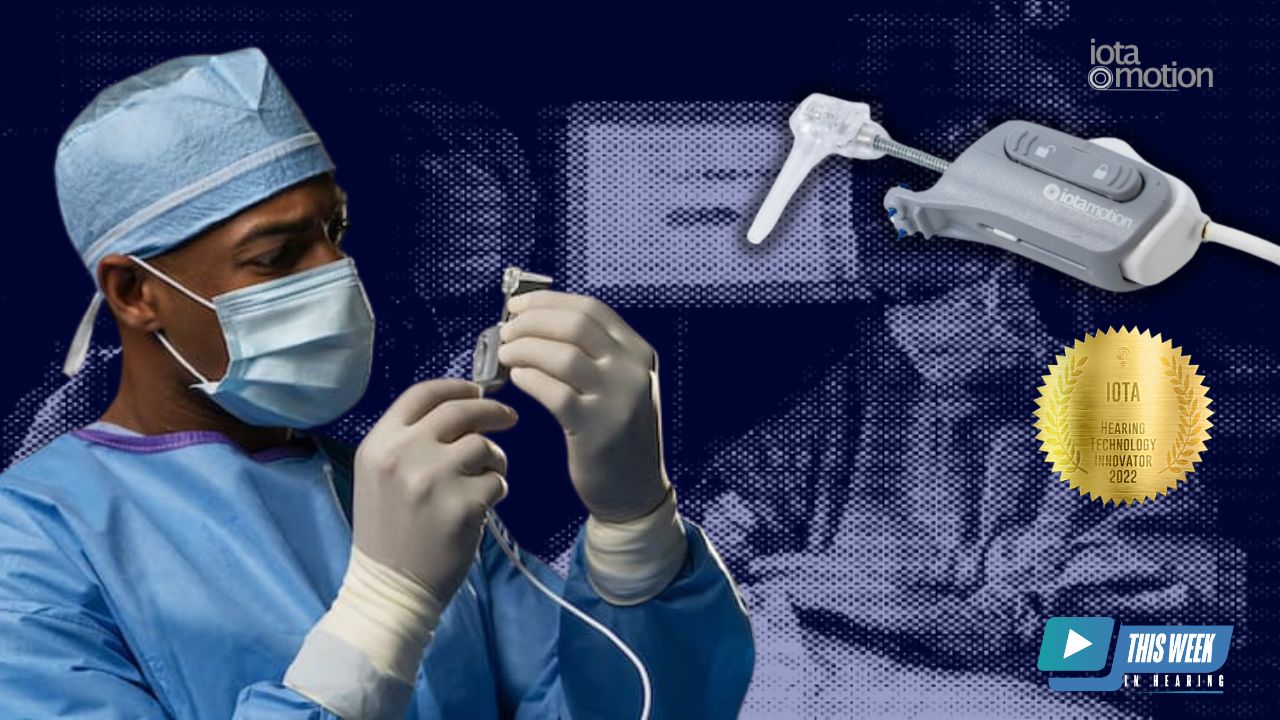
 The company was recently named ‘Innovator of the Year’ in the 2022 Hearing Technology Innovator Awards for its iotaSOFT Insertion System – a robotic-assisted insertion technology intended to aid surgeons in the placement of cochlear implant electrode arrays.
The company was recently named ‘Innovator of the Year’ in the 2022 Hearing Technology Innovator Awards for its iotaSOFT Insertion System – a robotic-assisted insertion technology intended to aid surgeons in the placement of cochlear implant electrode arrays.
The iotaSOFT system works by controlling the speed of insertion. The technology is a thumb-sized bone mounted robotic-assisted device that can be used with electrodes across implant manufacturers and was specifically designed for seamless integration in the operating room to enable the surgeon to insert the electrode beyond human capability.
Full Episode Transcript
Dave Kemp 0:10
Okay, everybody and welcome back to another episode of This Week in Hearing, today I’m joined by Marlan and Wade with iotaMotion, the winner of the 2022 Hearing Health Matters ‘Innovator of the Year’ award. So Marlan and Wade, thanks so much for being here, I want to give you guys an opportunity to talk all about iotaMotion and the technology that you’ve been developing. So why don’t we start maybe with a little bit of a background, we’ll start with you, Marlan. Just maybe a brief bio, and then how iotaMotion came to be. Well, thanks.
Marlan Hansen 0:44
Thanks, Dave, for having us, we really appreciate this opportunity. And so yeah, I’m a neuro otologist, which means I’m an inner ear skull base surgeon and spend a lot of my life dealing with patients with hearing loss and cochlear implants and an environment where we really have thought a lot about how we can best help patients take advantage of this technology. And so, you know, the motivation comes from the imperative to be able to put in a in an electrode array into a very small delicate structure, and not cause damage. And, you know, that becomes really important, as we see an expanding number of patients with hearing loss as we get an older population, as we see an expanding number of patients who can benefit from the, from the technology, as we see an expanding number of surgeons who needed to be able to do this, there’s a lot of things that need to happen in order for for every patient have a good consistent outcome with this technology into to get in so that people can benefit. I think a lot of that’s motivated what we did, a lot of it has to do with some of the reservations that people have. So as I was early on, we were doing these very, very short, hybrid cochlear implants that only went you know, maybe 10 millimeters into a cochlea whereas a standard would might go in anywhere from 20 to 30 millimeters into the cochlea. And they worked really well, because they save the patient’s low frequency hearing, they had really, they had a lot of benefit from combining this electrical stimulation with their residual acoustic hearing. But some of them go on to lose hearing. And for a variety of reasons. And then what happens if you just have a short electrode? And so that was another motivation is, you know, not only how do we enable these electrodes to be able to be placed very delicately and consistently, and so they caus as least traumas possible, but maybe even down the road, how do we then be able to adjust the position of the electrode in the cochlea, so that it matches up with that individual’s patient’s hearing needs and so kind of individualizing the technology for each patient. Those are kind of the ideas that were knocking around in my mind as we started to think about this.
Dave Kemp 3:23
Yeah, that’s really interesting. And so when you at the University of Iowa, when you started to kind of like, bring this thing to fruition- What was the big breakthrough for you? What was the aha moment, if you will, where you started to bring this to life? And then bring it ultimately into its current iteration?
Marlan Hansen 3:43
Yeah, so that’s, that’s great. There was a couple of critical, I would say steps along the way. The aha moment was actually many, many years ago, when I said this, this is really what we need to do. The challenge was, you know, I was busy doing a lot of other things. I had my own clinical practice, I have a lab that looks at other things as well. It was just busy time. So I really didn’t have anyone that I could dedicate or focus on it. And then Chris Kaufmann, who’s the co founder, the company came in, and he was in a program where he could work two years in my lab, and so I sort of pitched the idea to him and, and he does have a background in biomedical engineering. And he was very interested, he, I think, he saw the importance of what we were doing and, and really, he had the capacity to dedicate time and he had the expertise to work on it. And so really, it was Chris and I getting together I think was the first time we could actually try and materialize or put into actually action, some of the other thoughts that we had had. And so Chris was critical. And so you’ll, you’ll hear a theme that this has really been even though it’s idea based, it’s also people based. So Chris was a was a critical component. under that having an environment like the University of Iowa and my surgical colleagues here was a critical component of even some of the original ideas. The other critical piece was, as Chris and I were doing this, we early on got matched up with Eric Timko. And he really sat down with this and looked at it, not just from a concept, you know, this is a good idea. But what could you actually get into a or, and have, you know, an FDA approved product that actually a surgeon can actually use instead of sort of what Eric would have described as a science experiment. And so having his expertise in kind of bringing in knowledge around regulatory and what what’s feasible, what’s doable? What can we actually how can we actually materialize this not just from a concept, but actually something that can help patients. So that was another critical step was bringing Eric on board, getting his ideas around that. And then of course, his helping with some fundraising, and it takes money to kind of do these things. So I think those were the key leaps or steps that that kind of helped launch this.
Dave Kemp 6:12
Yeah, that’s super helpful. And I guess now would be a good time to just kind of like break down what exactly iotaMotion is, what iotaSOFT is. So I know that, you know, this is a robotic system more or less for cochlear insertion, but just help to kind of, for the audience, really paint a picture of what you’re trying to solve with this. And, you know, kind of like, how it compares to the status quo of the mechanical insertion. And, and maybe some of the upside of having this be done by robotics.
Marlan Hansen 6:50
Yeah, so maybe an equivalent is like, if you’re driving a car. And you know, that if you drive fast, then anytime you hit bump into something, you’re gonna cause more damage. And also, if you drive erratically start, stop, start stop that, that’s going to cause some problems. And, and the cochlea is kind of this very delicate, essentially, fluid filled space with these very thin membranes in it and those membranes, because it’s a closed space, or essentially a closed space, as you increase the volume of the cochlea, as you put in the electrode, you generate these incredible pressure spikes that need to be relieved or vented out, or else you’re just going to essentially rupture membranes or things like that. So what’s really manually what happens is surgeons have known I think, for a long time that it’s important to go really slow. So when we were trying to, for instance, in patients with really good hearing, and we’re trying to save their hearing, we would try and put it in as slow as possible. To limit the amount of trauma that we’re causing to limit these pressure spikes. The problem is in a human, even in the best surgeon’s hand, human kinetics are limited. And we basically do a lot of start and stopping and jittering on a micro scale, this is on a very small scale. And so the nice thing about robotics and what iotaMotion does, or iotaSOFT does, is it takes and allows that insertion to be done at a much slower speed than a surgeon can do. And much more consistently, so there’s not this variation, or spikes and peaks and things. And, and so that’s really what we’re trying to do the other I think critical component of when we thought about robotics, you know, this is really a tool built by surgeons, for surgeons, so we really wanted it to be something that would you know, kind of easily fit into the natural flow and, and, or, and solve a problem in the or rather than just having technology for technology’s sake, or robotics, robotics sake, or having engineers that don’t do the surgery, but come in and have these elegant, you know, big behemoth arms that do all these little motion things, but really couldn’t practically be adopted across lots and lots of centers and sites. And so we we tried to design it that way, where it would be something that that wouldn’t add, you know, three hours are lots of complexity to the case but could just achieve what we what we thought was really critical about the case consistently across a wide range of surgeons.
Dave Kemp 9:42
Yeah, like whenever I think of adding and augmenting you know, robotics into like, an OR, I do think of like the big mechanical arms and all that and this is just like a really small thing and I I liked what you said there too, that it’s kind of like, you know, designed by surgeons for surgeons. So it seems like and wait, I’ll bring you into the conversation now. It seems like one of the cool things about this is it very much is appealing to kind of like the whole current cochlear ecosystem, from the patients, to the surgeons that are actually fitting it to the companies that did you know, that making create the cochlear implants, and I know where you had been at Cochlear before, what brought you to iotaMotion? And what did you see that got you excited to come work for this company?
Wade Colburn 10:33
Yeah, so thanks for having us. Yeah. So, in my past at Cochlear, I was overseeing the implant, you know, system. And particularly, I worked pretty closely with Marlan and so obviously had relationship, you know, with him and understood what he was working on. And, and so, I had a brief stint actually at Bose looking at over the counter hearing aids. And, but, but have been in the hearing space for quite some time and really intrigued by, you know, the the impact that a cochlear implant can have on people’s lives. And I think, you know, Marlon alluded this a little earlier, but, you know, if you look at the data, there’s, you know, cochlear implants are severely under penetrated in the sense that a lot of people could benefit from them. And there is an expectation that more and more patients will have better and better hearing that, you know, I’m planning what they’re doing okay, but they’re not doing as good as they could be. And so I think this technology, you know, is really designed to fit in with all three cochlear implant manufacturers. So we’re compatible with MED EL, Cochlear, and Advanced Bionics electrodes, and the intent of it is to really be able to provide that consistency, you know, across all insertions for for the different implant manufacturers and across different surgeons, with the intent of trying to, you know, ultimately reduce the trauma to the cochlea, and, and subsequently, you know, maintain that greater confidence, both from the surgeon and also in the patients that of getting a cochlear implant. So, we know, one of the big barriers, you know, that people come is are, am I going to do better? Am I going to lose my residual hearing? You know, and, and we’re hoping that, you know, this is still, you know, a year in or so in terms of in the commercial market, and we’re in a limited market release. But, you know, we we like, what we’re seeing so far, and the centers that we’ve been at, and we’re continuing to, you know, look forward and see what, what, what shakes out, but yeah, I think for us, it’s, it’s, you know, Marlan probably speak to this a little bit, but there’s patients, you know, patients that were now coming asking for a robotic system. And I think that just telling of the impact robotics can have both in the wider medical field, but but also in cochlear implants. So, now mind, if you want to chime in on on any of that?
Marlan Hansen 13:09
Yeah, I mean, I think what you alluded to weight is, is something that that we have thought about, and I think is really critical is that there’s a an expanding number of patients who we need to be able to consider about receiving cochlear implants. And these, these patients have the highest demand in terms of being able to preserve their structure and function and give them benefit. So personally, what I want to see is 90% of patients get 90% word scores at, you know, 90% of the time. So I mean, we really want to be able to be at that level, that 90% level, where 90% of surgeons consistently get a good result 90% of patients get excellent, excellent, you know, not these flat outcomes that we’re dealing with now, but better. And that requires a lot of things that’s not just that requires that we implant healthier ears, that requires that, that when we do implant them, we maintain the function that’s existing there, and that we’re supplementing that function not replacing the function. So think of a cochlear implant, not just as a replacement for a dead ear, but think of as a supplement for a partially diseased here. And I mean, these are kind of the things that that motivate us, and I think where we have to be as a industry because until there’s hair cell regeneration or something like that, you know, hearing aids are always going to be limited because the end organ is just not healthy enough for the for the stimulation. So it takes a combined it takes both electrical and acoustic hearing. It takes preservation of what you have. And, you know, that’s what, that’s what’s really, I think, exciting and motivating and imperative about what what we need to do as an industry. not just out of motion, but as an industry.
Dave Kemp 15:03
Yeah. And I think it’s, it’s really exciting to that you’re seeing the candidacy requirements from the Medicare side of things are expanding. And so you know, like, there’s more people that are eligible for a cochlear implant. And like you said earlier, you know, we have an aging population. So, it seems like this is a great area to be innovating around. Because I think that there probably is, you know, it’s a really underserved market in terms of the proliferation not not enough people have been fit with these. And I think that a lot of that comes down to maybe the stigma that people may be associated with, like, what the process is, like getting implanted to begin with. And so if you can make that you can alleviate that, I think, for the for the prospective patient, that they know that there are these new methods of inserting the electrodes. And I just think that’s gonna really bode well for the appeal of this so that more people get on board with it, which coincides with, you know, kind of what’s happening from a financial standpoint as well.
Marlan Hansen 16:05
Well, as you tack on to a comment you made Dave is that you know, that as we, as you know, CMS just expanded the criteria as that criteria is expanding, then that places even more demand on surgeons, industry and things to be better at preserving things. Because we’re going in to healthier things, we’re not just fixing a completely dead here, we’re, we’re really going into healthy tissue here. But the other thing is there’s other technologies that are also coming out that are also exciting. I mean, we have now electrophysiology and you know, electrocochleography, where in an ear that’s receiving an implant, you can be putting a sound or an acoustic signal, and using electrodes on the cochlear implant or extra cochlear electrodes that record the function of that ear. And, and the nice thing is, if you can integrate that with robotics, then you have not only a sensing system, but you have a control system, it’s like an automatic braking system for your car where ‘oh boy a deer just ran in front of you’ and the brake stop and things like that, that are really going to be what’s required. So it’s going to be a marriage of a few technologies. And I think robotics can sit nicely, kind of at the core hub of that as being the control mechanism that whether you’re doing image guidance, or whether you’re doing electrophysiological feedback, or whatever you need that control to make those other sensors meaningful.
Dave Kemp 17:34
Did you have a background in robotics? You know, clearly you you kind of saw the problem. But how did you – It sounds like you said there were a lot of really important people along the way. How did the How did this kind of like form into a robotics company? I guess that was the inevitable path for what you were trying to do.
Marlan Hansen 17:58
That’s, that was what I could see. That’s what we needed to do. I have, I did not have any expeience, I come from a cell and molecular neurobiology background. And, and so that’s what I’m saying is I had ideas around this for a long time, but I didn’t have personal ability to do it. And I didn’t have capacity to, to, you know, do what really needs to be done. And that’s why Chris was so critical and important for, for what we’re doing is, you know, Chris showed up and one we had funding to, you know, he could work in the lab for two years, and we could support him. And so that was important. And he had the expertise and the drive the initiative and the ambition to do it. So
Dave Kemp 18:43
yeah, I just I feel fortunate that now I know your company and what you all are doing, I think it’s it’s super exciting. So maybe help to share with the audience and help us understand where you go from here. I know that there were I think there’s somewhere upwards of like, 20 of these insertions that you’ve done so far. Is it you know, what’s kind of the roadmap from here and what can we expect out of your company for the remainder of this year and into next?
Marlan Hansen 19:11
Well, I should say one of the things that Wade can maybe mentioned some cover this up too, but But bringing weight on board and people like Wade so in a way when I you know he’s outstanding and and having someone who has the expertise, knowledge of electrodes, the expertise, knowledge of the surgery, the expertise, knowledge of the implants, and even the surgeons and the environment in which they live and things like that. Having someone like that, that can be kind of in charge of getting our product and other surgeons hands is absolutely critical because he you know, he gets it he understands the little nuances of of not only how our device works, but of all the rest that’s going around cochlear implants from a surgical point of view. And so you know, it’s having the right team together. And I think we’ve been very fortunate that we have a great team that works on this. You know, so I mean, there’s there’s sort of the short term, the long term, and then the very, very long term. And I think, really what what we focus on now is, you know, the next few months or next six months, or whatever, and that is really getting, getting the technology out in the hands of more surgeons, I think now at Iowa, we’re probably closer to 60 cases, maybe I don’t know where we are somewhere along there. And Wade’s been out 5-6-7 centers across the US doing other cases. So the numbers are getting larger, and we learn as as it gets adopted. That’s why we limit, initially, we didn’t want to just release this out into the wild, we wanted to make sure that we could understand well, how it was performing. And how we can make sure that as we get this out into the hands of everybody that it’s going to be effective and work well for them. And that’s what Wade’s been so critical about doing. And that’s what we’re about the next, you know, six months is really just making sure we as a company have systems in place, and mechanisms that we can train and that the product is working as we expect it to work. And if we need to make little tweaks here and there things like that.
Dave Kemp 21:23
That’s awesome. So Wade, what, you know, boots on the ground, you’re actually in these, you know, interacting with the surgeons and stuff, like what has the feedback been like?
Wade Colburn 21:33
Yeah, so you know, the like Marlan said, we’ve we’ve been at about six or seven centers, we expect to be in probably 10 to 15, in the year and probably grow that to around 20 or 25. Next positive to date, in the sense of, you know, I think people some of the key things that people realize is it does provide stability, so you have both hands available now. And you can have a little more flexibility. So it provides stability of the electrode, I mean, obviously does control down to point one millimeters per second, which, you know, a normal typical surgeon is around one millimeter per second, potentially is kind of what they aim for. So, you know, when I say that, it means one thing, but when you actually see it, it’s a different situation. So I think people are oftentimes saying, oh, yeah, you know, I go with a really small slow insertion, and then they see it in their, in their surgery and or the nurse even in the OR sees it, and they’re, they’re looking on the screen, and they’re like, is it moving? And oftentimes, I have to ask the same question, because it’s, it’s going so slow, which is the point. You know, and and I think people are oftentimes impressed by just the fact of like, okay, yeah, this really does provide a consistent smooth insertion, which is what the goal is, you know, it provides that stabilization. And, you know, and I think also, you know, like you mentioned, or it was discussed earlier robotics, you know, I think get this wrap up, Oh, it’s this massive system, it’s going to add an hour, it’s going to add two hours. And, you know, sure, yeah, there’s a learning curve and the initial case or two, but, you know, once you do one or two cases, I mean, it’s, you know, 5-10 minutes, you know, to set the system up and get it in there. And that includes the actual insertion time, which, you know, the goal is to slow everyone down. Right, that’s, that’s the whole point. In that situation. So, you know, I think for us, it’s been good to learn, you know, we’re we’re already looking at ways we can make tweaks for version 2, you know, and, and how we can expand and also, like, Marlon mentioned how we can incorporate with other enabling technologies and other, you know, systems. But, but, you know, overall, it’s been a good start, you know, we’ve been fortunate to work with a couple really good Doc’s and, you know, surgeons across the country and a good centers, and we’ll continue to look for more partnerships of people that appreciate enabling technology, see the value of robotics, you know, see the value of slow atraumatic insertions and the impact that’s going to have on the industry across. And, you know, we know that, you know, with any new tech, it’s, it’s early adopters, first, you know, that are seeing that, and then we’ll, you know, continue to build the evidence, you know, of both clinical evidence and, and benchtop, good at categoric evidence and have, you know, both Iowa and other centers be a critical part of that. So we’re looking forward to what’s to come.
Marlan Hansen 24:32
I should mention, so Wade is Wade is a hard worker, he’s boots on the ground. He’s actually on the ground. He’s He’s covering cases and so he has to pull off the road. He’s not he doesn’t have a Texas drawl. Even though we have a Texas presence. That’s actually this is audio from the being on the road on a hotspot kind of thing. And with actual leaves falling and a real
Dave Kemp 24:55
set of a stone early in nature.
Marlan Hansen 24:58
He’s awesome. But he It brings up an important point that maybe we’ve touched on a little bit that I think is really, as we think about robotics, it really does enable a lot of these other technologies. And so, you know, some of the cool things that we’ve been able to do is like, we can go so slow that you know, electrocochleography becomes really, really meaningful now, yeah, or these trans impedance measures that that sometimes you want to make around electro that you would have to otherwise run once the electrodes into position, you can start to run things in real time. One of the interests and then Wade mentioned, you know, kind of the control that you have. So this was an interesting patient that I had just last week or week before where she was deaf in one ear, but could hear in the other ear. And we we did it where she was under local anaesthetic, she wasn’t completely asleep. And so we were putting sound into her good ear, as we put the electrode in very slowly robotically, and we’re stimulating the electrode. And we’re pitch matching to the acoustic signal in our other ear as we’re putting it in. And she could tell us, right, you know, she says, Well, that’s higher, that’s higher. That’s, that’s getting closer. Oh, that’s pretty close. That’s it. Okay, now, it’s lower than that. And so we could, we could do that all. And she was actually moving her head and doing these things. But it was the electrode was perfectly still because it secured her head. So even though she’s moving around and doing things, it worked out great. It was a lot of fun. I think the patient enjoyed doing it. And we’ve had a lot of fun, too. So
Dave Kemp 26:32
yeah, I just find this whole thing to be so fascinating, because, you know, it’s like, this is what robotics in medical setting looks like. And I think it’s so cool. But like it does, it unlocks things that I at least personally, I hadn’t originally thought of like, you know, super, super slow abilities that, you know, we as humans don’t have. So it’s like, what else can these things do? This seems to just be one very specific use case of something that probably can be applied to a whole range of other things.
Marlan Hansen 27:05
We hope so we think so.
Dave Kemp 27:09
Absolutely. Well, thank you two so much for coming on. iotaMotion. This is an exciting new company that we in the hearing health space, need to keep an eye on, I’m certainly going to be looking at everything that you all are doing. I just think it’s awesome that like this technology is conducive to, like you said with the three big cochlear implant companies. So I do think that this is going to be a name that we hear more and more of, and I’m really excited to see what you all do, because it feels like you’re just getting started.
Marlan Hansen 27:40
Thank you, Dave. Thanks for having us. It’s good to see.
Dave Kemp 27:43
Absolutely. All right, everybody. Take care.
Be sure to subscribe to the TWIH YouTube channel for the latest episodes each week and follow This Week in Hearing on LinkedIn and Twitter.
Prefer to listen on the go? Tune into the TWIH Podcast on your favorite podcast streaming service, including Apple, Spotify, Google and more.
About the Panel

Marlan Hansen, MD, FACS, is a co-founder and co-inventor of iotaMotion technology currently serving as the Chief Medical Officer. He is a respected leader and researcher within the cochlear implant industry based on his extensive clinical experience, publications and industry relationships. Dr. Hansen is an expert clinician-scientist trained in neurotology/skull base surgery and cell and molecular neurobiology. He is a Department Chair of Otolaryngology and a Professor of Neurosurgery and Molecular Physiology & Biophysics at the University of Iowa. Currently, his clinical practice focuses on both cochlear implants and surgery of the inner ear and skull base. He is active in research and publications, currently serving as the PI for a clinical trial funded by the Dept. of Defense to use hearing preservation electrodes to treat veterans with noise-induced hearing loss; and is co-investigator on the ongoing clinical trials at the University of Iowa that determine the outcomes achieved with hearing preservation cochlear implant surgery.
 Wade Colburn is a biomedical engineer turned marketer, who serves as the Director of Commercialization at iotaMotion. His responsibilities at iotaMotion involve overseeing the initial launch and utilization of the iotaSOFT® Insertion System. He partners closely with surgeon and audiology partners and is focused on leveraging robotics to expand the cochlear implant market. Prior to joining iotaMotion, Wade has been in the ENT industry serving as a product manager and engineer at Bose, Cochlear Americas and Cook Biotech. He has been a part of the design, launch and commercialization of multiple devices in use today. Graduating from North Carolina State in Biomedical Engineering, he leverages his early engineering experiences to ensure the best possible products continue to be delivered to the market.
Wade Colburn is a biomedical engineer turned marketer, who serves as the Director of Commercialization at iotaMotion. His responsibilities at iotaMotion involve overseeing the initial launch and utilization of the iotaSOFT® Insertion System. He partners closely with surgeon and audiology partners and is focused on leveraging robotics to expand the cochlear implant market. Prior to joining iotaMotion, Wade has been in the ENT industry serving as a product manager and engineer at Bose, Cochlear Americas and Cook Biotech. He has been a part of the design, launch and commercialization of multiple devices in use today. Graduating from North Carolina State in Biomedical Engineering, he leverages his early engineering experiences to ensure the best possible products continue to be delivered to the market.
 Dave Kemp is the Director of Business Development & Marketing at Oaktree Products and the Founder & Editor of Future Ear. In 2017, Dave launched his blog, FutureEar.co, where he writes about what’s happening at the intersection of voice technology, wearables and hearing healthcare. In 2019, Dave started the Future Ear Radio podcast, where he and his guests discuss emerging technology pertaining to hearing aids and consumer hearables. He has been published in the Harvard Business Review, co-authored the book, “Voice Technology in Healthcare,” writes frequently for the prominent voice technology website, Voicebot.ai, and has been featured on NPR’s Marketplace.
Dave Kemp is the Director of Business Development & Marketing at Oaktree Products and the Founder & Editor of Future Ear. In 2017, Dave launched his blog, FutureEar.co, where he writes about what’s happening at the intersection of voice technology, wearables and hearing healthcare. In 2019, Dave started the Future Ear Radio podcast, where he and his guests discuss emerging technology pertaining to hearing aids and consumer hearables. He has been published in the Harvard Business Review, co-authored the book, “Voice Technology in Healthcare,” writes frequently for the prominent voice technology website, Voicebot.ai, and has been featured on NPR’s Marketplace.