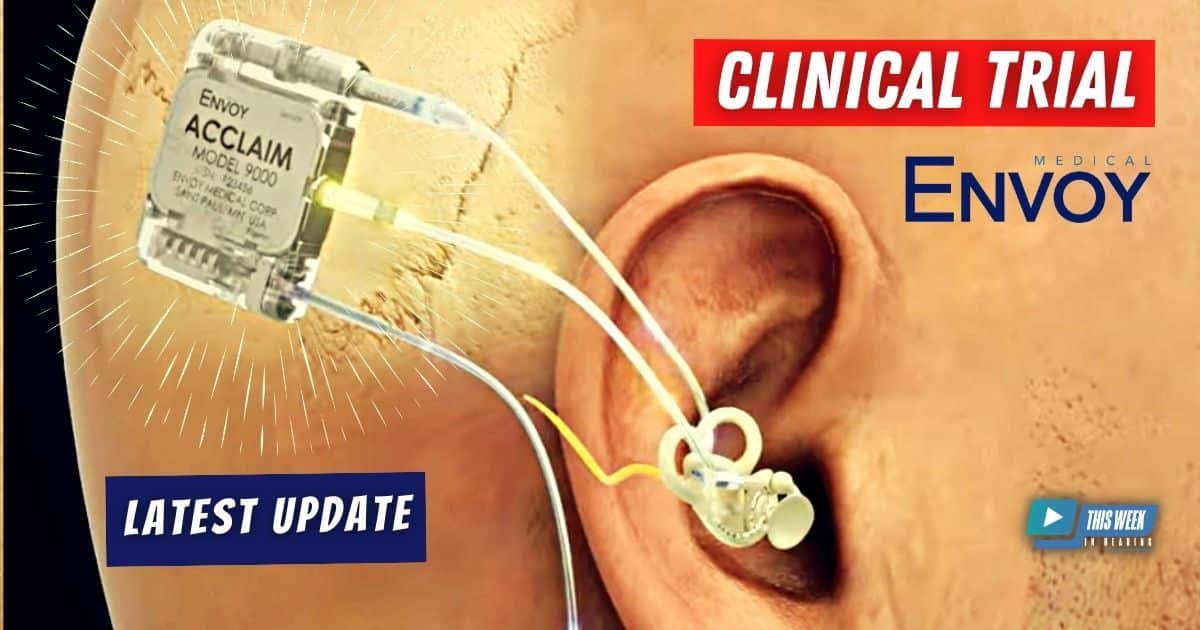
Brian Taylor revisits the topic of cochlear implants and sits down for a follow-up conversation with Brent Lucas, CEO of Envoy Medical. Expanding on their previous discussion from last year, the interview explores the company’s latest advancements.
The discussion highlights the outcomes of a clinical trial at Mayo Clinic involving the Acclaim fully implanted cochlear implant, shedding light on the surgical process, positive results, and unexpected findings. Notably, the Acclaim cochlear implant’s fully implanted design opens up the possibility for users to wear potentially wear a hearing aid on the same side, presenting an intriguing option for patients.
Brent Lucas also further shares insights into Envoy Medical’s recent listing on Nasdaq (COCH), emphasizing the company’s commitment to controlling its own destiny in the hearing implant industry.
For those seeking more information, Envoy Medical’s Acclaim implant and other technologies, visit the company’s website
Full Episode Transcript
Hello and welcome to another episode of This Week in Hearing. I’m Brian Taylor. And today our topic is cochlear implants. And with us to discuss some of the latest breakthroughs in cochlear implant technology is Brent Lucas, who’s the CEO of Envoy Medical. Brent, it’s been a while since we’ve had you on the broadcast. Welcome back. Thank you. I appreciate have the invitation back and update you on how things are going. Sure. And before we go any further, I’m guessing some people haven’t listened to that broadcast from about a year and a half ago. Maybe if you could kind of review for our listeners, who is Envoy medical and what’s your role there? Sure. So Envoy Medical is a hearing implant company based in White Bear Lake, Minnesota. We have two breakthrough technologies. Both are fully implanted. One is the Esteem hearing device, which is a fully implanted active middle ear hearing device. The other one is the Acclaim, which is the first fully implanted cochlear implant that uses no microphone whatsoever. And we are in a clinical trial at Mayo Clinic, a Minnesota Company. It’s great to work with Mayo Clinic in Rochester, Minnesota. Three patients implanted, and we are moving along in that process. We also recently listed publicly on the Nasdaq under COCH and look forward to more and more people becoming aware of Envoy and hopefully taking an interest in what we’re doing. Yeah, I see that on the screen there behind you. So let’s start with the trial with Mayo Clinic. I know that you recently published in the Journal of Clinical Medicine the results with three patients that you mentioned that were implanted with the acclaim product. Tell us a little bit more about that study and what the results were. Yes. So it was great to see the Mayo team put out a publication, and we look forward to seeing more of what they’re going to be talking about related to this study and other work that they’re doing. The study essentially touched on the surgical process to implant the acclaim and some of their learnings from the procedure. One of the criticisms that some of our competitors have suggested is that because it is fully implanted, it’ll be a significantly harder procedure. And I think that paper highlighted that a capable surgeon can quite – not, quote unquote, easy, but relatively easily put in the acclaim, and that shouldn’t be an impediment. They also touched on that there were no significant adverse events for any of the three patients. The surgeries went relatively speaking about as well as you can hope for, for a first in human study. And then we are. Anxious to follow up with some of the audiological results and quality of life results, but we have to be careful and let them do their process. I know it’s a long process. I’m sure the FDA is involved in that. Yeah. You have a protocol, right? And so we have a protocol that we’ve filed with our investigational device exemption, and we are following that protocol. But I can share some of the learnings from that so far that have been relatively interesting. That’d be great. Yeah. Too far into the specifics from a quality life standpoint. For example, we are using Teddy McCracken’s CIQOL 35, I believe is the official title of that, which is a quality of life tool used specifically for cochlear implants. So far, we’ve had two of the three patients that have been implanted have reported an increase in that. One of the patients had a slight decrease. We believe that slight decrease is probably related to some of the frustrating sort of pesky issues that we’ve had with the trial. Obviously, it’s a first in human study, an early feasibility study. These are designed to help you tweak the design as you go through the process. And so we found some areas of improvement. And I think one of the subjects has reported a little bit of frustration related to that process, which is fair. The other really interesting thing that happened, which was an unintended finding, was one of the patients, in the beginning, we have a slight signal to noise issue that we’re working through, which essentially is to say that there’s a little bit of noise in the system that’s getting picked up and coming into the signal, which is then not allowing us to really turn the device up all the way that we would like to. And so one of the patients, during his first activation visit through his hearing aid in his Acclaimed ear. So because our device is fully implanted and because we use the eardrum to pick up sound, you can put something in your ear. So I don’t have one, obviously, but I could be wearing my AirPod on top of my Acclaim ear. This individual put his hearing aid on top of his Acclaim ear, and the result was that he got quite good performance. He was satisfied with the device. So while we’re not going to have a combination product, we’re not planning on that being our end goal. It is very interesting for us to see that you can wear any hearing aid off the shelf, can go into your Acclaim ear, and you can get a little bit more out of the Acclaim. So we think that’s a very interesting finding that we hope to find out more about as we go for further. The other thing, just to talk about on that. Is some of the other TICIs, if you will, are totally implanted cochlear implants that are being discussed. Talk about an external mode and an invisible hearing mode. By the way, Envoy Medical has the trademark for ‘invisible hearing’, but anyway, that’s what they call their non external mode. And so this is an opportunity for us to say, well, the Acclaim is fully implanted, and we can also have an external mode if need be. A patient can also use a hearing aid on top of their device if they would like, if they would so choose. And we think it’s a pretty interesting opportunity because as a medical device manufacturer, it can be very hard to stay up to date with the latest and greatest trends in connectivity, Bluetooth, commercialization of sort of consumer products. Right. So if we can just build the base and have the Acclaim be the real internal structure, and then a patient can use whatever they want on the outside, in their ear, if they so choose. I think that’s an interesting wrinkle that we didn’t really think we were going to find in this process. Yeah, that’s really interesting. I think most of our listeners are familiar with the bimodal arrangement with a hearing aid on one side in a cochlear implant on the other. And now we have this new possibility where they could be wearing a device on the same side as a cochlear implant. That sounds pretty intriguing. Yes. And I think, look, we talked about this last time, and it’s well discussed in the other publications and other presentations from the other manufacturers. The penetration rate for adults in the US for cochlear implants is very low. And part of that, we believe, is the requirement for an external in the traditional cochlear implant. But there’s also the topic of patient awareness, and can they use other things on top of their ear? There’s also the incentives of a retail audiologist, frankly. And so this is an interesting wrinkle in where a retail audiologist can refer on a cochlear implant candidate, but also maintain that patient as a hearing aid user if they so choose. That’s a great point. I think that, in my opinion, one of the reasons why the penetration rate is relatively low for cochlear implants and adults is that many hearing aid professional hearing aid fitters are sort of reluctant to give up those patients or maybe are not familiar with the criteria. So I think adding this wrinkle is really beneficial for everybody. No, I think that’s great. That’s definitely a consideration that we’re excited to be able to bring that forward as an. Exactly. Exactly. So the second thing I wanted to ask you about, and I think you can tell from your screen, is Envoy Medical is now traded on NASDAQ. Tell us about that process. And. What that means to the future of Envoy Medical. Sure. It’s been an interesting process, for sure. And one of the things I’ve been most surprised by is how uneducated the American investor base is in hearing companies, in part because there aren’t a lot of, if really any anymore, I believe Eargo just went private again a couple of days ago, if I’m not mistaken, how few hearing companies are publicly listed in the United States on a major exchange like the NYSE or the Nasdaq. So one of the things that’s been a little surprising for me, but I’m very excited to be doing is educating the investor public, retail investors, institutions, and others on the hearing industry, but specifically the hearing implant industry. But that has been an interesting wrinkle of this process, too, is really starting off sort of educating people on what is hearing loss and what is the spectrum of hearing healthcare solutions, who are the players, who’s involved. So, yes, I have the background, says Nasdaq COCH, because we want people to go out there and follow us and see what we’re doing and if they like what they see, take part in our future. Outside of that, we went through what’s called a de-SPAC process. So we essentially merged with a SPAC. There’s a lot of legal jargon around it, but essentially the SPAC was already publicly listed. We merged with that SPAC and then now control the entity through the process. We have a new board, a new corporate board with some people from our private board. Whitney Haring-Smith, who was the CEO of the SPAC, will now also be on our board. And then we have three new independent directors, which we’re very excited to be having sort of an outside, fresh take on things. So, yeah, we’re really excited. Look, it’s not all sunshine and Rainbows and any of this stuff, but one of the reasons Envoy wanted to do this, to be frank with you, is we wanted to be able to control our own destiny. And when you are a startup company trying to disrupt a business, you need to either look for the big players in the industry to sort of take you out, right, as an exit strategy, or you need to figure out a way to control your own destiny. And for us, this was a way to say, look, we’re here, and we are going to be a major player in this industry, and we’ve been here for 28 years right now, I believe. And so we look for being here for another 30 and hopefully becoming one of the market leaders. That’s great. I’m with Brent Lucas, CEO of Envoy Medical. Brent, where can people learn more about envoy medical and some of the things you’re working on, the products that you mentioned? I’m guessing you have a website. Yes, EnvoyMedical.com is where you can find information about our Esteem device and our Acclaim device. The Acclaim device is still investigational and not for commercial sale, but the Esteem device is a commercially available product. If you’re a patient or an audiologist or a surgeon, we have all sorts of information for you there. Follow us on social, we’re active on LinkedIn, and we also will be publishing more and more press releases related to our ticker symbol – COCH. If you know, on your phone, if you want to follow along and get the latest and greatest press releases related to that, follow that ticker symbol and you’ll be able to hear more from us. That’s great. Thanks for the update, Brent, on all the things happening at Envoy Medical. Hope to catch up with you again in another year or two. Yeah, hopefully have some more exciting things to talk about. Sounds good.
Be sure to subscribe to the TWIH YouTube channel for the latest episodes each week and follow This Week in Hearing on LinkedIn and Twitter.
Prefer to listen on the go? Tune into the TWIH Podcast on your favorite podcast streaming service, including Apple, Spotify, Google and more.
About the Panel
 Brian Taylor, AuD, is the senior director of audiology for Signia. He is also the editor of Audiology Practices, a quarterly journal of the Academy of Doctors of Audiology, editor-at-large for Hearing Health and Technology Matters and adjunct instructor at the University of Wisconsin.
Brian Taylor, AuD, is the senior director of audiology for Signia. He is also the editor of Audiology Practices, a quarterly journal of the Academy of Doctors of Audiology, editor-at-large for Hearing Health and Technology Matters and adjunct instructor at the University of Wisconsin.
 Brent Lucas is the Chief Executive Officer of Envoy Medical Corporation, based in White Bear Lake, Minnesota.
Brent Lucas is the Chief Executive Officer of Envoy Medical Corporation, based in White Bear Lake, Minnesota.