This week, Amyn Amlani sits down with Dr. Marcus Atlas, founder of the Ear Science Institute Australia, to discuss the institute’s groundbreaking work, particularly the development of ClearDrum.
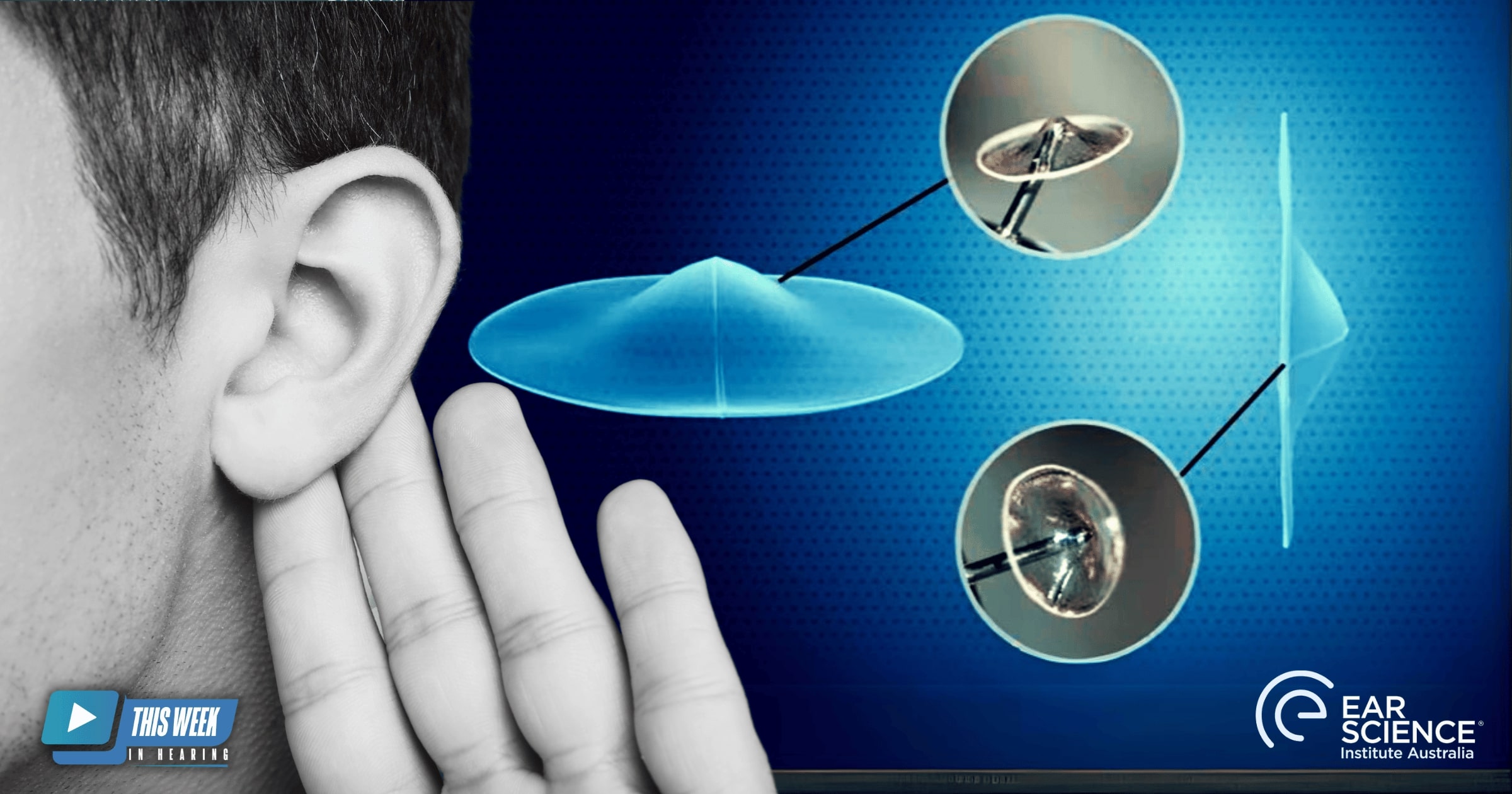
ClearDrum is an innovative biosynthetic prosthetic designed to reconstruct the human tympanic membrane using silk fibroin. Unlike traditional graft materials like cartilage, ClearDrum offers transparency, superior acoustic properties, and robust mechanical strength, addressing challenges like chronic ear disease and surgical monitoring. This innovation exemplifies the institute’s focus on integrating clinical practice with cutting-edge research to improve patient outcomes.
During the conversation, Dr. Atlas also sheds light on the challenges of bringing an idea from the lab to the clinic, underscoring the years of meticulous research and collaboration required to develop ClearDrum. With FDA approval and clinical trials on the horizon, the prosthetic is poised to set new standards in tympanic membrane repair, potentially transforming hearing care globally.
Dr. Atlas highlights the Ear Science Institute’s multifaceted approach, combining clinical services, research, and community engagement. The institute’s Lions Hearing Clinics not only deliver audiological care to Western Australia’s remote areas but also reinvest profits into research initiatives like ClearDrum.
Full Episode Transcript
Welcome to another issue of This Week in Hearing. Today, I’m joined by Professor Marcus Atlas, a surgeon scientist based in Perth, Australia. Thank you for your participation and let us start by having you talk a bit about yourself and the Ear Science Institute of Australia. Excellent. Thanks for inviting me. I’m very grateful. This is a great opportunity to tell the story, I think, to audiologists in the U.S. I’ve already had the experience of being able to tell the story of ClearDrum to some of my US colleagues at the Trilogical Society meeting some years ago. But this is a great opportunity. Ear Science Institute Australia, which is what I founded over 20 years ago, is medical research institute, collaborating with major universities here in Australia dedicated entirely to ear and hearing disorders. And we’re driven by innovation. And the innovation is not just in the research world, it’s also in the clinical world. So our foundations are upon the delivery of clinical services which are particularly audiological, but also in the area of implantation, cochlear implantation as well as implantation of all of the implants and ear surgery, as well as our activities in research, our activities in education and our activities in the community and that forms ear science. And ear science is very much focused on the ear, of course. And so medical research institutes that are just focused in one particular area are not really that common in the world. They’re usually allied in a bigger organization and sometimes losing their focus. So we believe this is a wonderful thing to be able to attach our clinical ideas to our science ideas. And so that is where we spawned ClearDrum, which is a biosynthetic, synthetic prosthetic method to reconstruct the human tympanic membrane. Well, I find it fascinating. So I worked for a ear, nose and throat physician a number of years ago and I’ve been working part time now with one. And I had a conversation with him earlier today and I was asking him, and I said, you know, 20 years ago I recall that the middle ear surgeries were just about every third or fourth surgery that you did. And I asked him, I said, has that stayed the same or has it changed over time? And interestingly enough, at least here in my little world outside of Dallas here, he has said that the otological surgeries that he does has actually increased. Are you finding that to be similar in Australia? I’ve been, I’ve been an ear and skull based surgeon, so an otologist- neurotologist for nearly 30 years. And so all of my work is in that area. So I don’t see a proportion changing from the nose and throat component. But what I do see is a change in complexion of this, the subspecialty of otology, neurotology. And there’s been a drastic move to implants. And so we’re seeing, and that really is of audiological interest because the world of implantation has just drastically increased as we see implantation occurring of patients who previously we wouldn’t even have considered previously, single sided deafness, lots of low frequency sensory neural hearing loss where patients weren’t traditionally considered to be implant candidates. So that has changed. But in terms of tympanic and middle ear surgery, what I’ve noticed and what I’ve seen evolve is reconstruction of the middle ear utilizing acicular prostheses in chronic ear disease has reduced significantly. And the reason was, and you know, this is because the results of a ossiculoplasty, unless the patients had very specific indications and had very specific good prognostic findings, the results of middle ear reconstruction aren’t particularly good. And those patients, if they had a dry ear, would do better with their hearing aid, often. And now they were either doing better with a hearing aid or a bone conduction implant than they were with multiple attempts at reconstruction of the ossicular chain in severe chronic ear disease. So that has changed. So implants have changed, I think the indication for middle ear reconstruction of the ossicles, but of the tympanic membrane. There hasn’t been much in terms of what’s been available to us to reconstruct the tympanic membrane with a synthetic repair. As you know, it’s always been allografts, temporal fascia, cartilage and other materials that we get from the patient’s body to reconstruct the human tympanic membrane. And we saw that as being the problem. Yeah, yeah, no, thanks for clarifying that. Its fascinating in the role of the audiologist in the middle ear piece. Before we move on to anything else in Australia, how does that play out. The role of the. I missed that part of that question. I’m sorry, the role. Yeah. So the role of the audiologist. So here in the States we do preoperative audiometric testing. Then the surgery occurs and we do post operative testing. And then if there’s an implant or a device or whatever, the audiologist is involved. I’m assuming it’s the same in Australia, or maybe there’s something else that you all are doing that is novel that we should be aware of? No, I think our systems for our audiological training and the way audiologists interact with otolaryngologists and particularly in implants units, is very similar. It’s very similar. I have lots of friends both in audiology and otolaryngology in the States, and you do things very similar to us. Similarly to us. I think what I’ve seen evolve though, is what I’ve said is this implant story as being very much part of a multidisciplinary way to reconstruct hearing. We feel so strongly about this at Ear Science, this idea that we should try and treat our patients with hearing disorders in a multidisciplinary way with multiple devices that change over the patient’s life. What we can offer to children now is different to what. To what we offer adults. We need to do this in a multidisciplinary way. So the way hearing aids, implants and surgery interact together, it used to be in otosclerosis, for instance, we would consider a stapedectomy. And in more advanced otosclerosis with an evidence of cochlear hearing loss, they would get a hearing aid after successful stapedectomy. So there would be this evolution of the way we would rehabilitate a patient with otosclerosis. Now this happens in nearly all of our patients because implants are possible in a patient who is getting recurrent otitis externa or. This evolution of the way we treat patients means that you guys and us as surgeons really need to work together effectively for a really good patient outcome. That I think has changed in Australia and I suspect in the States as well. Yeah, no, I agree. And I think one of the things, and I was really impressed by your website is the whole area of education and awareness. And I really liked how your website kind of built out the opportunities and not only having the literacy for the patient, but also for the professional. And you know, in the States we typically tend to only have the literacy for the professional. And on the patient side, it’s there, but it’s really not as vibrant as it could be. And so can you talk a little bit about how that developed? I think that’s a really important component. What we’ve noticed is that our patients are driving a lot of referrals to us. So people, particularly in the audiological world, they can come off the street to one of our Lions hearing clinics and there’s 30 Lions clinics around Western Australia. The Lions hearing clinics are associated with the Ear Science Institute Australia that we own, the Lions Hearing Clinics. And this way is. This is our method to reach out into the community with audiological services. And those patients are now coming to see us because they’ve seen things online or they’ve empowered themselves with this new information. And so there’s so much has changed in audiology in terms of what’s possible for patients with hearing aid fittings, with implant fittings, with, with surgery plus implants, implants plus surgery hearing aid devices after successful otosclerosis surgery. They know about this and they’re general practitioners who are the guardians of health care in Australia. That’s a little bit different to the States in terms of how you access medical health care in our countries. But they often don’t know. They simply don’t know what’s available in hearing rehabilitation services. So the patients empower themselves and we help them empower themselves. And so the. I don’t remember the number, but it’s a significant percentage of, particularly our implant patients are coming in because they’ve read about something or they’ve learned about something from a patient, from a family member, not from their medical practitioner. And so this is, this is sort of the way we’re communicating with the community now and we’re really delivering this information, you know, this podcast. There’ll be patients who will see this podcast and because they want to know, they want to know about reconstruction of the tympanic membrane. They want to learn about new ways. It’s remarkable, I think how this has evolved during my time as a surgeon and I’m sure in your time as an audiologist. Yeah, no, absolutely. And I think one of the things that also intrigues me, and I was reading a little bit about this on your site, if I remember my geography correctly, you’re on the western side of the, of Australia. And so there’s accessibility issues because people have to drive distances. If I read this correctly, there’s also some remote care that you all take, that you all provide to your patients, given the accessibility issues that they face. Can you talk a little bit about that? Because in the States, that really hasn’t taken off like we had hoped it would. You know, it’s interesting, I’ll give it a sort of a flavor of the states, because remote healthcare in Australia, particularly in ear disease, is very much influenced by our indigenous population. Our indigenous population have terrible ear disease. They have terrible ear disease. It’s a scourge of children living in remote parts of particularly Western Australia. But in Australia, that they get a lot of chronic ear disease, tympanic membrane disease, chronic middle ear discharge, hearing loss, and social disadvantage because of this. And in a prosperous country like mine and yours, that that exists in Australia, it’s a terrible indictment on all of us, despite all of our work. So that brings us to what you were talking about is the delivery of hearing care out of the major cities. And Western Australia is this huge state. And so how do we deliver that? And we deliver it usually hands on. And of course, telehealth has given us this opportunity to try and deliver in a different way. And so we’ve really worked hard at the delivery of telehealth. Audiology, Otolaryngology can be delivered utilizing telehealth. There are difficulties and there are logistical problems associated with audiology as well as surgery. But there’s a lot that we can do with telehealth to bypass the difficulty of getting specialists and clinicians into these faraway communities. And so that’s what we’ve done. So we have outreach programs where we fly to various parts of our big state and deliver both audiological and surgical services. But we’ve tried to improve the delivery of hearing health, particularly with audiology, telehealth, and we’ve developed a number of devices that allow us to be involved in remote care, remote audiology. But in Australia, kids all get their hearing aids and the majority of their hearing services through a government subsidized group. And so that kind of limits some of the things we can do in terms of delivery of hearing aids which some of these kids really need. So it’s interesting how technology has interfaced with delivery of hearing services particularly in remote Australia. Yeah, well thanks for sharing that. Just changing topics here. So again as I was looking on your website, getting ready for this, this interview here, I noticed two things. Something called the Lions Hearing Clinic and something called the Ear Science Surgical Facility. Can you talk a little bit about those components? Lions Hearing Clinics Traditionally the Lions founder clubs of Western Australia have been involved in hearing healthcare for over 50 years. And when I first came to Perth to take up the foundation Chair in Otolaryngology at the University of Western Australia some 24 years ago, they came to me not long after I arrived with two clinics that they were running. And it seemed to me that this was a wonderful way for the Lions International to be involved in hearing care in a more progressive, more advanced, more up to date way. And so we took these clinics from them and expanded them. And that’s been the way we’ve delivered audiological hearing services around the whole of Western Australia. Some 30 clinics now seeing tens of thousands of patients a year. I think 40,000 appointments last year and to see patients around Western Australia. So that’s the delivery of hearing services through the Lions Hearing Clinics and they run as a business and all of the, the profits from the business of audiological services go back into research, go back into audiological research and into research like ClearDrum. So we are a not for profit organisation. And the Lions Hearing Clinics run as a really active hearing aid provider or hearing services provider because of our involvement in all sorts of devices as well as hearing aids. Ear Science Surgery Facility is the way our surgeons interact with the institute. Our surgeons I operate as independent contractors and they deliver all of their hearing services within the building that I’m that I’m sitting in now where the surgical facility provides access to outpatient care to all of the clinics that we deliver and we also have operating rooms here in this facility. So it’s whole of hearing care at Ear Science. And we’ve got this beautiful building that I’m in which delivers clinical care. And then not too far from here all of our research is within a university and hospital facility where we do all of our basic science research. So that’s, that’s essentially Ear Science Institute Australia. Yeah, that’s, that’s really interesting. And so we’ve talked about the clinical side. Let’s talk a little bit about the research side. So you were saying that you have a partnership with the, with the institution and can you talk a little bit about how the two interface and then some of the research that’s taking place as well. Ear Science. We’ve developed over the years five major themes that we’re involved in and one of them is clinical and we’ve talked a little bit about that where we’re doing research in our implant patients, particularly in our hearing aid patients and our surgical patients and looking at clinical outcomes, research delivery, new databases, new ways to be able to gather clinical information and publish that. And you’d be familiar with that. Of course the basic science part of this also involves some more basic science in implant research where we’re looking at electrocochleography, intraoperative hearing testing. That group’s run by Christo Besta and it’s particularly interested in automation of ways to look at electrophysiologically at an implantation using ECoG as well as other ways to monitor implantation and outcomes and prediction of outcomes in implantation. Then there’s another group that is involved in ClearDrum and that is the silk and its various products in the way that we can rehabilitate the middle ear and the tympanic membrane and ClearDrum, which we’ll talk about I guess in a few minutes time is spawned out of that group and then there’s a hearing therapeutics group and that course of research is particularly into the area of hair cell regeneration, organoids, cochlear organoids, where we’ve been able to create a three dimensional organoid which is a human cochlea in a petri dish essentially that replicates cochlear function and that we can experiment on. We’ve got an Usher organoid. So the Usher syndrome, hearing loss and genetic abnormality associated in Usher is transferred into organoid and that’s what we’re doing a lot of work on and trying to regenerate the hair cells, particularly in Usher syndrome and various other genetic hearing losses. So our big move, and I think the big move for all of us in the future is genetic hearing loss nearly a significant number of hearing loss in our implant cochlear implant patients is related to genetic hearing loss and of course, all of the genetic hearing losses that we know about. And of course, the exciting news this year in otoferlin where otoferlin genetic hearing loss has been treated successfully using gene therapy techniques, both beginning in China and now in other areas in the world, including and including a group in the States who are working on that. So it’s really interesting. Genetic hearing loss really interests us because we see potential interventions in genetic hearing loss being able to impact not just a small group of hearing loss patients, but a really large group of hearing loss patients. So they’re our group groups. They’re our exciting groups at the Ear Science Institute research areas and they are really exciting areas of hearing research. I think I’m excited to be part of hearing research these days. There’s been lots of advance, particularly in the last 10 years. It’s been fun. Yeah, no, absolutely. And I can hear your passion. And you’re absolutely right. You know, the whole issue with looking at genes and the ability to regenerate and restore and all these other things, I think is really, really going to be an exciting area that we’ll hopefully get to apply in clinic here, you know, within the next decade or so. But it’s coming pretty quick. So thank you for sharing that. You mentioned ClearDrum. Why don’t you talk a little bit about that? Because. Was actually the reason that we were going to have this discussion. So why don’t you have a chat about that with us? Thanks for asking. ClearDrum It takes a long time. I really wanted to tell you the fact that it’s taken a really long time, because some of my otolaryngology colleagues in the States are going to be saying, Marcus was talking about this about 10 years ago. What on earth happened? I thought he died. And the problem is taking something from – as a surgeon, I think often we don’t realize, maybe as audiologists too, is that everything we do is based in science. You know, all the discoveries we’ve made have come from somebody having an idea and developing something from a basic interest, using basic science and scientific techniques to create something, to create an audiometer, to create surgical techniques, and the operating microscope. And all of these wonderful things that are part of our everyday life come from this wonderful science. And. But being a surgeon is a much more pragmatic thing. And so you go about your life in applying the science, but in a really translated way. So a new thing in my life was to take something from the bench to the bedside, to take it all the way from an idea to something that could be translated into our clinical world. And that is my excuse for it taking so long, because it is such a difficult thing to do. And we began in a collaborative way with a material science group at Deakin University in Victoria in Australia. And they’re this wonderful group that are involved in materials and the research into various materials. Carbon fiber for tires and all sorts of materials. But one of their interests was in silk. And they were using silk for textiles and various other things. But they knew that silk could be used in biological ways in biotechnology and in biomedical applications because it had very specific characteristics that allowed it to be used in biomaterials. And of course, you know, that we’ve used silk sutures for 100 years. So there’s a history of the use of silk. And so we worked with them to take silk from animals, from silkworms, and change it into a material by removing the saracen. And what remained was a thing called silk fibroin and silk fibroin. You could do all sorts of things to it. It’s completely transparent, and you can make it into foams, into fibers, into membranes. And that’s what we did. We made it into a membrane, and we determined its suitability for reconstruction of the tympanic membrane. And we knew that it had to have specific mechanical qualities, it had to have specific acoustic qualities. It had to be transparent because the tympanic membrane is basically, as, you know, transparent, can see into the middle ear. But all of the things we used to use for reconstruction of the tympanic membrane, they were not transparent. You could not see into the middle ear cartilage, particularly it used to obstruct the eardrum. And you remember seeing pieces of cartilage that had been grafted into the tympanic membrane. You couldn’t see anything through them. And so you couldn’t monitor the ear after surgery because the cartilage would get in the way. So we had all of this in our minds as we started to create these silk membranes which had specific mechanical qualities, they were transparent, and they had really specific acoustic qualities, which I guess is of interest to you and your audience. And because the materials that we typically use, temporal fascia and cartilage, don’t have the specific acoustic frequency qualities of the human tympanic membrane. And so we worked really hard at that and replicating the acoustic qualities of a normal human tympanic membrane with silk, which we did, and particularly also on mechanical qualities, because, as you know, the ongoing problems of middle ear disease and negative pressure and eustachian tube dysfunction, they continue on after we reconstruct the tympanic membrane. And that’s why we often use cartilage, because it has stronger mechanical strength to resist the negative pressure that draws in the tympanic membrane and creates retraction pockets, cholesteatoma and negative pressure inside the middle ear. So we worked at particular mechanical core qualities that would be, that would be suitable for reconstruction of the tympanic membrane and then something that could come off the shelf. So in the operating room, we spend a lot of time harvesting these grafts, harvesting temporatus fascia from the temporalis muscle, or harvesting cartilage from the tragus or the pinna. And so utilizing something that was off the shelf and available to us in unlimited quantities as opposed to autologous grafts, was very appealing. And so that is what the premise for ClearDrum was and what we finally, finally produced, which is now going through the FDA ringer, as we try and get FDA approval and then of course, a clinical trial which is planned, I hope as soon as we get FDA approval to do so. Well, that’s really, really fascinating and it’s hats off to you and your team for Being innovative and pushing the envelope here so that one, it’s easier for the surgeon and then number two, the outcomes for the patient are also enhanced. So thank you to you and your team for doing that is just tremendous. Thank you. Yeah, it’s wonderful. But it takes work and time and it’s really been when our professional lives intermixed with this completely new experience of taking something from, from the lab into translating it into clinical life. It is quite an experience, quite a journey. Quite a journey. So do you have a kind of an estimated timeline on when you think there’ll be the ClearDrum will potentially be available for application in your everyday surgery operating room? Ballpark is we hope to be to complete if everything goes to plan to complete our clinical trials or at least submit for FDA approval following the clinical trials in 2027. But, but I keep giving dates and they continue to change. It’s terrifying to give dates and, and I keep getting told not to give dates for all sorts of commercial reasons. But, but I’m a scientist, so. And everyone wants to know you know, we really need to know. So it looks like end of 2027 when hopefully it more widespread clinical use. Well, I again, that’s congratulations to you. I look forward to seeing that. I’ve got lots of friends that are ear nose and throat physicians. I’ve got two that are otologists. And I told one of them. Yesterday – Friday that we were going to have this conversation and she says she’s very familiar with your work. And that’s the question that I just asked was on her behalf, by the way. You have, you have an audience, at least in the US that is eager to, to be able to apply these creations that you’re having. So just, just know that I did. And I’m very grateful that you know, they’re kind words and you know, many people ask why did we go to the fda? Why are we going to the US and there’s lots of reasons. It’s a huge market and it’s a wonderful it’s sophisticated way to deliver hearing services. But it’s mostly because I’ve got so many friends in the States and who show exactly what you said. Show interest, show innovation. They’re really interested in how to do things better. And so I really admire that quality about your friends and the healthcare service in the US So that’s why. And I hope we can bring it as soon as possible. Well, again, I look forward to seeing it. I know that you know, she was excited that we were going to have this conversation. And you know, again, thank you for all the innovation that you do, because without it, you know, we can’t move forward, not only with the care that we give, but the patients can’t get better with their abilities to hear. Because, as you pointed out, some of the older methodologies, like the cartilage, just didn’t produce the outcomes that we were. That they intended to do. But at least it was a start. And it’s not how you start, as my father would say, it’s how you finish. Well said. That’s true. That’s very true. So thank you. Thank you very much for your support and also for your interest in what we’re doing. And we’re really excited about that and many of the other things we’re doing at Ear Science. And if we get a chance to be able to speak about some of the other things in the future, that’ll be wonderful, too. No, absolutely. And what we’ll do is for the audience, we’ll be sure that we have your website and you know, any assets and things that you care to share particularly to this topic, and then maybe some others we’ll be able to highlight for them so that folks that are interested can then dive in and say, you know what? Let me get a little more information about this or that. So. And your contact information, or at least your team’s contact information, we’ll have that readily available as well. Thank you. Thank you for all of that. It’s wonderful speaking to you. You as well, Marcus. Thank you so much for your time. I know it’s early where you are. You’ve got patients to tend to. I just got home from work, so it’s been a long day and. Uh-huh. Thanks again for your time. Wonderful. Thanks for that. Thanks for that. See you again soon, I hope. Yes, S.
Be sure to subscribe to the TWIH YouTube channel for the latest episodes each week, and follow This Week in Hearing on LinkedIn and on X (formerly Twitter).
Prefer to listen on the go? Tune into the TWIH Podcast on your favorite podcast streaming service, including Apple, Spotify, Google and more.
About the Panel
 Professor Marcus Atlas is a globally respected ear and hearing implant surgeon and the founder of the Ear Science Institute Australia. With a career spanning decades in otology and neurotology, he has pioneered advancements in cochlear implantation, middle ear surgery, and hearing health innovation. Professor Atlas is committed to integrating research and clinical practice, as exemplified by the development of ClearDrum, a silk-based prosthetic designed to revolutionize tympanic membrane repair. His leadership has established the Ear Science Institute as a global center for hearing care, combining research, education, and community outreach to deliver cutting-edge solutions and improve patient outcomes
Professor Marcus Atlas is a globally respected ear and hearing implant surgeon and the founder of the Ear Science Institute Australia. With a career spanning decades in otology and neurotology, he has pioneered advancements in cochlear implantation, middle ear surgery, and hearing health innovation. Professor Atlas is committed to integrating research and clinical practice, as exemplified by the development of ClearDrum, a silk-based prosthetic designed to revolutionize tympanic membrane repair. His leadership has established the Ear Science Institute as a global center for hearing care, combining research, education, and community outreach to deliver cutting-edge solutions and improve patient outcomes
 Amyn M. Amlani, PhD, is President of Otolithic, LLC, a consulting firm that provides competitive market analysis and support strategy, economic and financial assessments, segment targeting strategies and tactics, professional development, and consumer insights. Dr. Amlani is currently the President-Elect of the Academy of Doctors of Audiology (ADA) and has been in hearing care for 25+ years, with extensive professional experience in the independent and medical audiology practice channels, as an academic and scholar, and in industry. Dr. Amlani also serves as section editor of Hearing Economics for Hearing Health & Technology Matters (HHTM).
Amyn M. Amlani, PhD, is President of Otolithic, LLC, a consulting firm that provides competitive market analysis and support strategy, economic and financial assessments, segment targeting strategies and tactics, professional development, and consumer insights. Dr. Amlani is currently the President-Elect of the Academy of Doctors of Audiology (ADA) and has been in hearing care for 25+ years, with extensive professional experience in the independent and medical audiology practice channels, as an academic and scholar, and in industry. Dr. Amlani also serves as section editor of Hearing Economics for Hearing Health & Technology Matters (HHTM).