Although the FDA has yet to officially codify the Over-the-Counter (OTC) Hearing Aid Act, signed into law in August 2017, one subcategory of amplification device, self-fitting hearing aids (SFHA), has been sold online by a couple of companies for some time.
According to published reports, for an amplification device to be classified as a self-fitting hearing aid, it should enable the user to:
- Physically insert the device into the ear canal after the appropriately sized ear tip and tube length has been determined.
- Complete a user-directed hearing test, conducted in-situ.
- Conduct an automated first-fit process, based on a prescriptive fitting formula.
- Fine tune and train the electroacoustic properties of the device during real world listening experiences. It are these attributes, with artificial intelligence and machine learning at their core (covered in more detail by Chris Heddon) that make OTC hearing aids a sustainable product category.
In essence, SFHAs are hearing aids prescribed or selected for the user, by the user. Self-fitting hearing aids could be sold directly, off-the-shelf, to consumers via websites or retail stores, or they could be prescribed to patients by licensed hearing care professionals during a routine clinical appointment.
The Appeal of Self Fitting Hearing Aids
There are several reasons SFHA could be appealing to consumers.
- In rural areas or places around the world where there is a shortage of hearing care professionals, SFHAs could be a way to address issues involving access of hearing care services.
- SFHAs might be a low-cost alternative for people who don’t have the financial means to purchase traditional hearing aids and its accompany service from a licensed professional.
- From a psychological perspective, adults who are ambivalent about using traditional hearing aids, purchased through the traditional clinical pathway might find SFHAs to be a low risk alternative they could try from the comforts of home.
Additionally, there could be benefits for providers who offer SFHAs as an option. It’s not hard to imagine providers identifying viable SFHA candidates and allowing them to self-direct their care. Thus freeing up time to see other patients, those with more complex cases, or simply spending more time addressing the emotional and social needs of patients, rather than time-consuming adjustments to hearing aids that some patients could complete independently with a SFHA.
Technology Advancements Driving Incremental SFHA Improvements
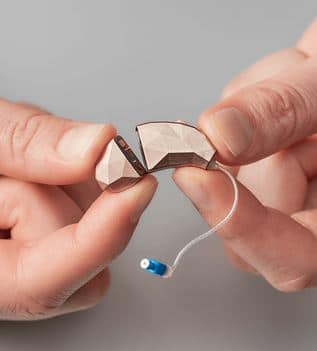
The Facett, by Blaumey Saunders, is a modular self-fit hearing aid
The convergence of two different types of technology has enabled SFHAs to emerge as a viable option for consumers. The first is the evolution of user control within conventional hearing aids.
It wasn’t all that long ago that it was a big deal for hearing aid users to select between different pre-adjusted settings to suit different listening situations. Since the introduction of these multi-memory hearing aids, more than 20 years ago, users can permanently change their hearing aids’ settings using trainable algorithms, while some current amplification technology allows the user to adjust and fine-tune a full range of acoustic parameters, often using a smartphone and an accompanying app.
Technological progress has also enabled sophisticated amplifiers to be packaged into devices that look like consumer audio devices, rather than hearing aids. This is the second factor driving SFHA innovation. Sometimes called hearables, many of these products have multi-tasking capability that allow the user to stream music, talk on their cell phone in a hands-free manner, plus provide the user with amplification. It is this combination of complete user control of the programming and fine-tuning process, along with form factors that don’t resemble traditional hearing aids that have the potential to disrupt the traditional clinical service delivery model.
Clinicians and patients alike need to know if SFHAs are effective. Next week, in Part 2, we will take a closer look at the existing research behind SFHA devices.
 Brian Taylor, AuD, is the director of clinical audiology for the Fuel Medical Group. He also serves as the editor of Audiology Practices, the quarterly journal of the Academy of Doctors of Audiology, and editor-in-chief of Hearing News Watch for HHTM. Brian has held a variety of positions within the industry, including stints with Amplifon (1999-2008) and Unitron (2008-2015). Dr. Taylor has more than 25 years of clinical, teaching and practice management experience. He has written and edited six textbooks, including the third edition of Audiology Practice Management, recently published by Thieme Press. He lives in Minneapolis, MN and can be reached at [email protected]
Brian Taylor, AuD, is the director of clinical audiology for the Fuel Medical Group. He also serves as the editor of Audiology Practices, the quarterly journal of the Academy of Doctors of Audiology, and editor-in-chief of Hearing News Watch for HHTM. Brian has held a variety of positions within the industry, including stints with Amplifon (1999-2008) and Unitron (2008-2015). Dr. Taylor has more than 25 years of clinical, teaching and practice management experience. He has written and edited six textbooks, including the third edition of Audiology Practice Management, recently published by Thieme Press. He lives in Minneapolis, MN and can be reached at [email protected]
*featured image courtesy i4u







