Oct. 24, 2023
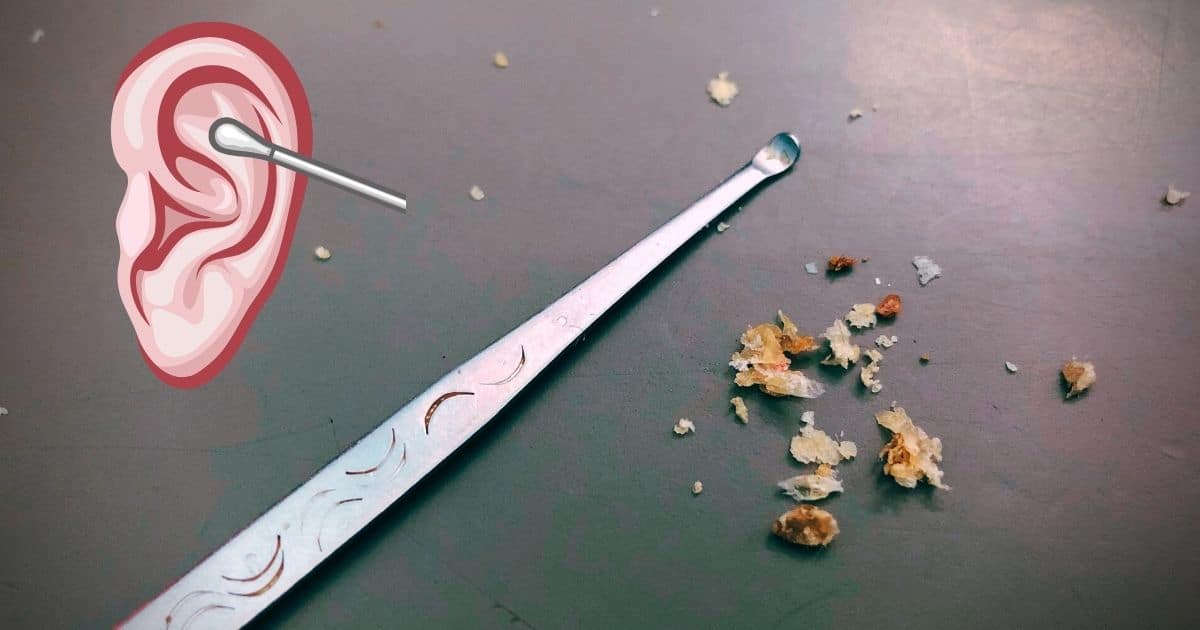
A few years ago, a trip to the city of Chengdu, China (home of the giant panda), provided an unexpected experience – the practice of ear-picking, or what some might refer to as ear cleaning or ear scraping. Ear Picking and Giant Pandas – Chengdu, China Most tourists who travel to Chengdu, China go to see the giant pandas. However,