Aug. 03, 2016
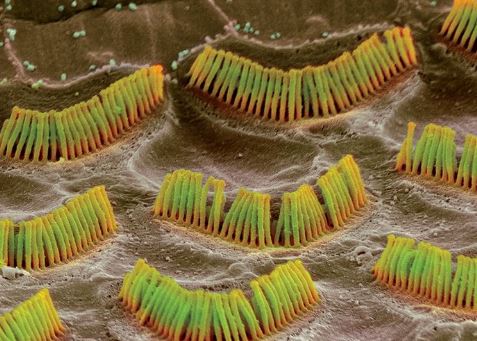
ZUG, SWITZERLAND — Biopharmaceutical company Auris Medical Holdings announced late last month that the US Food and Drug Administration (FDA) granted Fast Track designation to its investigational drug, KeyzilenTM (AM-101). Keyzilen is esketamine gel for intratympanic injection, intended for acute peripheral tinnitus following cochlear injury or otitis media in adults. The Fast Track designation by the FDA of an investigational drug helps expedite the