A bone-anchored hearing aid (BAHA), also known as a bone-anchored hearing implant (BAI), emerges as a transformative solution for individuals ineligible for conventional hearing aids. Unlike traditional hearing aids inserted into the ear canal or worn behind the ear, a BAHA is affixed to a soft band on the head or a metal implant surgically inserted into the skull.
Although the terms BAHA and BAI are used interchangeably, both referring to the same device, the medically precise term is BAI.
- History of Bone Conduction Hearing
- Types of Bone Conduction Hearing Systems
- How It Works
- Bone Conduction Candidacy
- Surgical vs. Non-Surgical Systems
- Surgical Procedures and Follow-Up
- Post-Surgery Considerations
- Advantages and Disadvantages
- Differences Between Cochlear Implants and BAHAs
- Market and Manufacturers
- Apps and Accessories
- Costs and Insurance Coverage
Bone Conduction Hearing – A Historical Perspective
The concept of bone conduction hearing dates back to the 1500s, credited to Girolamo Cardano. Early attempts to aid hearing involved using rods or spears to transmit sound vibrations to the ear. Significant advancements occurred in the 1900s with the development of carbon microphones, leading to the creation of bone conduction devices that turn sounds into vibrations on the mastoid bone behind the ear.
These early devices were bulky but paved the way for modern bone-anchored hearing aids. In 1977, Anders Tjellström introduced a percutaneous titanium device that allowed direct contact between bone tissue and titanium implants, a process known as osseointegration.
In the 1980s, these devices became commercially available, offering new solutions for people with conductive hearing loss (CHL), mixed hearing loss (MHL), and single-sided deafness (SSD).
Today, a growing number of people have become familiar with the bone conduction process through the use of bone conduction headphones which have become more widely available in recent years.
Types of Bone Conduction Hearing Systems
There are three types of BAHAs:
Softband Baha
A Softband BAHA is a type of nonsurgical bone conduction hearing aid. This device features a sound processor positioned against the mastoid area, held in place by a headband worn around the head. Unlike other BAHA types, it eliminates the need for surgical implantation, making it ideal for children under age 5 and individuals who either cannot undergo surgery or prefer to avoid it.
Providers often recommend the Softband BAHA for children whose skulls are not fully developed, offering a temporary solution until they are old enough for potential surgical options. It is also suitable for people who have specific conditions limiting surgical options. While this device provides the significant benefit of avoiding surgery, its applicability may be restricted in certain cases, and its effectiveness can vary depending on individual circumstances.
Some systems may also offer the option to attach directly to the skin with adhesive, providing flexible, non-invasive options for hearing enhancement.
Abutment BAHA:
Abutment BAHA refers to a type of Bone-Anchored Hearing Aid that involves a surgical procedure. It includes an implant inserted through the scalp and a connecting abutment that protrudes from the skin. This design aims to provide enhanced hearing outcomes compared to non-surgical options.
One of its notable advantages is MRI compatibility. However, users should consider the potential risk of complications at the implant site, which can include issues such as infection or skin irritation. The decision to opt for an Abutment BAHA involves weighing the benefits of improved hearing against the potential risks associated with the surgical procedure. (example of abutment BAHA show below)
Magnet BAHA:
Magnet BAHA is another category of Bone-Anchored Hearing Aid that incorporates a subcutaneous magnet, involving a surgical insertion. The external sound processor is attached to the magnet, offering a connection without a protruding abutment. This design aims to lower the risk of complications at the implant site compared to abutment-based systems.
However, users should be aware of potential challenges related to MRI compatibility, as well as the possibility of achieving slightly less favorable hearing outcomes compared to abutment-based alternatives.
The decision to choose a Magnet BAHA involves considering the trade-offs between surgical risks, hearing outcomes, and device compatibility with medical procedures like MRI scans.
Choosing the Right Fit:
While each BAHA type offers distinct advantages, the choice depends on individual needs and considerations. Abutment BAHAs excel in hearing outcomes and MRI compatibility but entail a slight risk of complications. Magnet BAHAs reduce implant site risks but may have MRI challenges and potentially less favorable hearing outcomes.
The softband BAHA provides a non-invasive option, but its application may be limited based on specific circumstances.
How it Works:
Traditional hearing aids amplify sound by directing it through the ear canal and past the middle ear. In contrast, bone conduction leverages the ear’s natural ability to perceive sound transmitted through the bones of the skull. A BAHA operates on the principle of bone conduction, eliminating the need for any device component to be inserted into the ear canal.
In contrast to traditional hearing aids, which transmit sound through the middle ear, this method requires no part of the device to be placed in the ear canal. These devices capture sound and transfer it through bone vibrations directly to the cochlea, bypassing the outer and middle ear entirely and creating a new hearing pathway. Bone conduction hearing aids can be used for one or both ears, and they may be combined with traditional hearing aids on the opposite ear if needed.
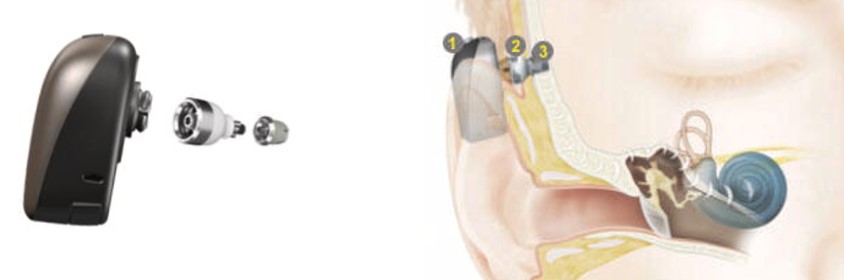
LEFT: Cochlear BAHA processor with abutment shown. RIGHT: 1) A sound processor detects sound and transforms it to vibrations. 2) Abutment connects the sound processor to the implant and 3) Implant transfers sound vibrations directly to the cochlea. Image credit: Cochlear
Components of a BAHA include a titanium implant, an external connector, and a sound processor. Surgically inserted BAHA devices are also known as osseo-integrated bone conduction devices.
Bone Conduction Hearing Aid Candidacy:
In the process of determining eligibility for a BAHA, individuals undergo a thorough evaluation to assess the type and degree of their hearing loss. Those who meet the criteria proceed to another appointment with an audiologist to explore the available devices and undergo a trial. During this session, the audiologist administers tests to evaluate the individual’s capacity to perceive sounds and comprehend speech using various types of hearing aid devices, aiming to identify the most suitable option tailored to their specific needs.
Potential candidates for bone conduction hearing aids may have experienced hearing loss due to various factors, including:
- Malformation of the ear canal or middle ear: Malformations such as narrowing of the ear canal or a malformed or absent pinna (external ear) can lead to conductive hearing loss. Typically congenital, these malformations can be addressed with a bone-anchored hearing solution that directly delivers sound vibrations to the inner ear by being in direct contact with the skull bones.
- Infection of the ear canal resulting in chronic draining ears: For individuals with chronic ear infections leading to fluid drainage from the middle ear into the ear canal, wearing traditional hearing aids may pose challenges. A bone-anchored hearing device, not obstructing the ear canal, offers an alternative solution.
- Congenital atresia: Outer ear malformations causing sound flow occlusion can be corrected with surgery. However, until a child is old enough for the surgical procedure, wearing a bone conduction hearing aid on a headband can stimulate the cochlea.
- Middle ear dysfunction/disease: When the middle ear system fails to transmit sound effectively, it becomes necessary to bypass this system for sound to reach the cochlea.
- Single-sided deafness (SSD): SSD occurs when one ear loses all hearing while the other has normal or slight hearing loss. This condition makes it challenging to determine sound direction (localization) and hinders speech comprehension in noisy environments. While a special pair of hearing aids (CROS device) can route sounds from the poorer hearing side to the better hearing side, a bone-anchored hearing system may be preferable for its discreet use of a single device.
- Ear allergies or irritation/annoyance from standard hearing aids: Individuals experiencing irritation in the outer ear canal due to allergies or standard hearing aid usage may find relief with a bone conduction device, which offers amplification while bypassing the outer ear canal.
Surgical or non surgical system?
When considering the use of bone conduction technology, the choice between surgical and non-surgical systems depends on various factors. Non-surgical devices, worn on a headband or attached directly to the skin with an adhesive, provide a less invasive option. On the other hand, surgical devices comprise an internal component and an external processor, requiring a more complex procedure. To make an informed decision, the otologist and audiologist will discuss the candidacy requirements for both systems, considering the medical history, degree of hearing loss, and personal preferences.
During the evaluation appointment, a trial with a bone-anchored processor connected to a headband may be conducted. This trial aims to provide
the patient with a firsthand experience of how sound delivered through bone conduction would sound in his/her specific case.
Non-Surgical Devices:
Nonsurgical devices are adaptable solutions, suitable for wearing on a headband or attaching directly to the skin with adhesive. This option is particularly beneficial for children who are not eligible for surgical procedures due to their still-soft skulls or for adults with mild hearing loss or those unable to undergo surgery.
Surgical Devices:
Surgically implanted devices consist of two components: an internal and an external part. The internal component is placed beneath the skin and within the bone behind your ear. Depending on your hearing needs, the audiologist or otolaryngologist may recommend either a percutaneous or transcutaneous internal component.
- Percutaneous Option: This involves a titanium post protruding through the skin, with the external processor attaching to the post.
- Transcutaneous Option: In this case, the internal component attaches to the external processor using magnets that work through the skin.
While there is no upper age limit for bone conduction, children must be at least five years old (or 12 years old for specific device types) to undergo the surgical procedure. The decision between surgical and non-surgical systems is a collaborative effort between the medical team and the patient, ensuring the chosen solution aligns with the unique needs and circumstances of the patient.
Surgical Procedures and Follow-Up:
Surgery:
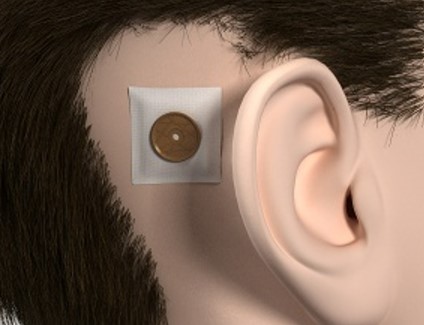
Image credit: Oticon Medical
The duration of bone-anchored hearing aid surgery typically ranges from 30 to 60 minutes, contingent on the specific type of system chosen. In the absence of contraindications or complications, the procedure allows for same-day discharge. The steps involved in bone-anchored hearing aid surgery include:
- Administration of anesthesia for patient comfort, with the type depending on individual circumstances (local or general anesthesia).
- A small incision behind the ear to access the surgical site.
- Creation of a small hole in the mastoid bone, located directly behind the ear and forming part of the skull.
- Placement of a small titanium implant in the mastoid bone, measuring approximately three to four millimeters in diameter.
- For BAHA with abutment only: Attachment of an abutment (connector) to the implant, with the connector slightly protruding for eventual skin healing.
- Closure of the incision using stitches and the application of surgical dressing.
In the case of the BAHA with the abutment, osseointegration is crucial for bone-anchored hearing aids, during which the bone fuses with the titanium implant to establish stability. This fusion is a prerequisite before attaching the sound processor to the external connector. The healing period varies depending on the chosen type of BAHA and individual healing capacity, typically spanning one to three months.
Post-Surgery: Device Activation:
Following the healing period, there is a required interval before activating your bone conduction hearing aid(s). Upon successful post-operative check-ups, the patient will return to the audiologist to receive the external component and initiate device activation.
Follow-Up:
Approximately one month after device activation, there will be a follow-up appointment with the audiologist. Subsequent periodic appointments will be scheduled for adjustments and upgrades to the external processor, ensuring optimal performance and addressing any evolving needs or preferences the patient may have. These follow-up sessions are essential for the ongoing care and maintenance of the bone-anchored hearing system.
Post-Surgery Appearance and Considerations for BAHA Recipients:

Image credit: Oticon Medical
After undergoing surgery for a bone-anchored hearing implant, individuals often express concerns about their appearance and well-being following the procedure.
It is reassuring to note that the surgery to place the implant in the bone behind the ear is typically minor which is easily hidden by hair or the external processor is matched to the patient’s hair or skin color.
What to Expect:
A bone conduction hearing device provides amplification without the need for an ear mold in the ear canal, offering enhanced comfort, especially for those prone to discomfort or infections in the ear. Some individuals report a more natural sound compared to conventional hearing aids due to the absence of an ear mold.
While these devices do not restore hearing to normal, they significantly facilitate managing everyday situations.
Advantages:
Bone-anchored hearing aids offer several advantages, including:
- Enhanced Comfort: No need to insert anything into the ear canal, resulting in a more comfortable fit and reducing the risk of skin irritation.
- Optimal Sound Quality: The bone-conducting signal is not dampened by the skin, leading to improved sound quality.
- Predictable Results: Trying a nonsurgical BAHA during an office visit allows individuals to anticipate outcomes post-surgery.
Disadvantages:
The primary drawback of bone-anchored hearing aids is the necessity for surgical placement. While BAHA surgery is minimally invasive, it carries inherent risks, including:
- Inflammation
- Infection
- Implant Failure: Occurs when the bone fails to fuse properly with the implant.
Additionally, individuals with bone-anchored hearing aids should be cautious of head trauma, as it could lead to more severe consequences such as infection, implant failure, or the need for repeat surgery. To mitigate this risk, wearing a helmet is advisable during activities involving contact sports or while biking or riding a motorcycle.
Understanding both the advantages and potential risks can help individuals make informed decisions about bone-anchored hearing implant surgery.
The Difference Between Cochlear Implants and BAHAs:
A Bone Anchored Hearing Aid (BAHA) transmits soundwaves to the inner ear, where skull vibrations assist in hearing. The device is placed behind the ear on the mastoid bone, offering a comfortable and non-intrusive option.
Cochlear Implant: A cochlear implant directly stimulates the auditory nerve, bypassing the inner ear, essentially the cochlear. It is beneficial for individuals with inner ear-cochlear damage, providing a solution to these types of hearing challenges.
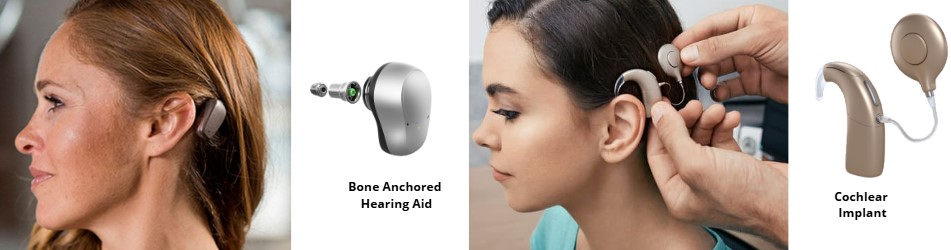
Market and Manufacturers:
There are various bone conduction hearing systems available, with FDA-approved options from leading manufacturers like Oticon Medical, MED EL, and Cochlear Americas. The technology is continually evolving, necessitating discussions with otologists and audiologists to determine the most suitable option based on medical history and hearing loss.
Manufacturers:
Cochlear Limited:
-
- Cochlear is renowned for cochlear implants and bone-anchored hearing aids (BAHA).
- The latest offering is the Baha 6 Max Sound Processor, introduced in 2021, featuring improved IP rating, extended frequency bandwidth, and direct streaming compatibility with both Apple and Android devices.
- Cochlear’s Osia System is an implantable hearing device with an active osseointegrated steady-state implant, introduced in 2019.
- Features include a slim and lightweight sound processor design, SmartSound iQ signal processing, and direct wireless streaming for compatible Apple products.

Oticon Medical:
-
- Oticon’s Ponto is a bone-anchored device, with the latest model being the Ponto 5.
- Ponto 5 is the smallest on the market, offering full wireless capabilities. It can be worn on a soft band for specific wearers and conditions.
- Oticon Sentio System: A transcutaneous bone conduction hearing solution that includes the Sentio Ti Implant, designed for surgical flexibility and adaptability to progressive hearing loss, and the Sentio 1 Mini Sound Processor, which provides wide bandwidth sound and a lightweight, discreet design. It received FDA and European clearance in July 2024
- Ownership of Oticon Medical was partially acquired by Cochlear, but regulators prevented the company from also taking its bone conduction business, so while the company no longer manufacturers cochlear implants, it continues to offer bone conduction devices

MED-EL:
-
- MED-EL’s BONEBRIDGE is a fully under-the-skin, active bone conduction implant introduced over ten years ago.
- The SAMBA 2 audio processor offers great sound quality and is MRI compatible at 1.5 Tesla without surgery.
- MED-ELs ADHEAR utilizes non-implanted bone conduction technology, providing great hearing without surgery.
- Designed for those with conductive hearing loss or single-sided deafness, it offers comfort and natural sound quality with no skin pressure.

Medtronic plc:
-
- Medtronic is a global producer of various medical devices, including bone conduction solutions.
- Ownership of the Sophono bone conduction hearing systems was transferred to BHM, an Austrian company, in 2023.
BHM-Tech Produktionsgesellschaft mbH:
-
- BHM specializes in non-surgical bone conduction solutions, including bone conduction hearing glasses.
- Their products focus on comfort, reliability, technical innovation, and excellent hearing experience tailored to individual needs.
- The Sophono Alpha 2 MPO ePlus offers uncompromised sound with a rechargeable system, up to 32 hours of use, and feedback-free gain compared to other transcutaneous devices.
Understanding the differences and advancements in these technologies ensures individuals can make informed decisions about the most suitable hearing solution for their unique needs.
Apps and Accessories – Enhancing the Bone-Anchored Hearing Experience:
Compatibility: Accessories and apps designed for bone-anchored hearing device users closely resemble those used by traditional hearing aid wearers and are commonly referred to as assistive listening devices. These include:
- Audio Streamers:
- Devices worn around the neck or as clips.
- Enable wearers to stream sound from TVs, cellphones, or music players directly into their sound processor.
- FM/DM Receiver:
- Useful in educational settings, tapping into school FM systems.
- Streams a teacher or lecturer’s voice directly into the wearer’s processor, especially beneficial in crowded or noisy venues.
- TV Streamers:
- Connect to televisions or computers, allowing users to wirelessly stream audio directly into their BAHA for enhanced clarity and speech comprehension.
- Smartphone Apps:
- Depending on the sophistication of the bone-anchored hearing system, apps can assist users in learning about and controlling their device’s volume and functions through their cell phones.
- Apps are typically provided free of charge by manufacturers like Oticon Medical or Cochlear Americas, available on Google Play™ or Apple® App stores.
- Direct Streaming:
- Depending on your phone type, some bone-anchored hearing devices may stream directly without additional accessories.
- Consult your audiologist to explore these options during device selection.
Costs:
While the exact surgical cost varies based on the procedure and individual factors, the average ranges between $10,000 and $17,000. Additionally:
- Sound processor prices range from $5,000 to $8,000 based on the chosen manufacturer and device features.
Insurance Coverage:
- Bone-anchored hearing systems and cochlear implants fall under the statutory Medicare benefit provision for prosthetic devices.
- Medicaid coverage for implantable hearing devices varies by state so it is important to check with the individual state’s Medicaid program for details.
Conclusions
The continual evolution of bone conduction hearing technology has led to devices that optimize signal transmission, reduce complications, and enhance hearing rehabilitation. Active transcutaneous devices such as the Osia® and Sentio™ systems leverage advanced signal transmission, efficient bone conduction, and reduced skin issues.
As with traditional hearing aids, a personalized evaluation by a professional hearing care provider is crucial to determine the best solution for your individual hearing and lifestyle needs.
References:
- Hopkins Medicine. (n.d.). BAHA: The Implantable Hearing Device. Retrieved from https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/baha–the-implantable-hearing-device
- Ellsperman, S. E., Nairn, E. M., & Stucken, E. Z. (2021). Review of Bone Conduction Hearing Devices. Audiology Research, 11*(2), 207–219. https://doi.org/10.3390/audiolres11020019
- Cleveland Clinic. (n.d.). Bone Anchored Auditory Implant. Retrieved from https://my.clevelandclinic.org/health/treatments/14794-bone-anchored-auditory-implant
- Great Ormond Street Hospital. (n.d.). Bone-Anchored Hearing Aids (BAHA).Retrieved from https://www.gosh.nhs.uk/conditions-and-treatments/procedures-and-treatments/bone-anchored-hearing-aids-baha/
- Oticon Medical. (n.d.). What You Need to Know About Bone-Anchored Hearing. Retrieved from https://www.oticonmedical.com/hearing-and-hearing-loss/new-to-bone-conduction/what-you-need-to-know-about-bone-anchored-hearing
- Ray, J., Wanees, E., Dawoud, M.M., et al. (2023). Evaluating the effectiveness of bone conduction hearing implants in rehabilitation of hearing loss. European Archives of Oto-Rhino-Laryngology, 280*(12), 3987–3996. https://doi.org/10.1007/s00405-023-07889-y
- Mudry A., Tjellström A. Historical background of bone conduction hearing devices and bone conduction hearing aids. Adv. Otorhinolaryngol. 2011;71:1–.
- Tjellström A., Lindström J., Hallén O., Albrektsson T., Brånemark P.I. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am. J. Otol. 1981;2:304–310.
- Brånemark P.I., Hansson B.O., Adell R., Breine U., Lindström J., Hallén O., Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast Reconstr. Surg. Suppl. 1977;16:1–132.
- Dun C.A.J., Faber H.T., de Wolf M.J.F., Cremers C.W.R.J., Hol M.K.S. An overview of different systems: The bone-anchored hearing aid. Adv. Otorhinolaryngol. 2011;71:22–31.
- MED-EL. (n.d.). BONEBRIDGE bone conduction implant system. Retrieved from https://www.medel.com/en-us/hearing-solutions/bonebridge#Bone-Conduction
- Cochlear. (n.d.). Cochlear Baha system. Retrieved from https://www.cochlear.com/us/en/home/products-and-accessories/cochlear-baha-system/baha-implant
About the Author
 Nausheen Dawood is an experienced Audiologist and Project Manager with a professional background including primary health care, corporate social investment, and business development. Proficient in the development of academic courses, training, and lecturing, with a focus on clinical student training and supervision. Adept in freelance copywriting, particularly in audiology and health-related topics. Holds a Masters degree in Audiology (Cum Laude), with a strong foundation in clinical research, project development, and strategic planning, complemented by technical training. Specializes in content development and training tailored to diverse audiences. Demonstrates a long-term commitment to research and development, including the implementation of randomized controlled trials, projects, and clinical examinations. Known for establishing robust networks and cultivating valuable stakeholder relationships.
Nausheen Dawood is an experienced Audiologist and Project Manager with a professional background including primary health care, corporate social investment, and business development. Proficient in the development of academic courses, training, and lecturing, with a focus on clinical student training and supervision. Adept in freelance copywriting, particularly in audiology and health-related topics. Holds a Masters degree in Audiology (Cum Laude), with a strong foundation in clinical research, project development, and strategic planning, complemented by technical training. Specializes in content development and training tailored to diverse audiences. Demonstrates a long-term commitment to research and development, including the implementation of randomized controlled trials, projects, and clinical examinations. Known for establishing robust networks and cultivating valuable stakeholder relationships.






