WHITE BEAR LAKE, MINNESOTA – Envoy Medical® Corporation, a hearing health company focused on providing innovative technologies across the hearing loss spectrum, today announced the first patient has been enrolled and implanted in the Acclaim® Cochlear Implant early feasibility study at Mayo Clinic in Rochester, Minnesota.
The surgery marks a key milestone for Envoy Medical on the road toward bringing the Acclaim Cochlear Implant to market.
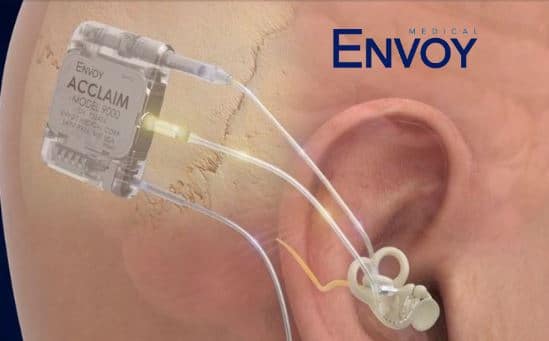
If approved by the FDA, the Acclaim would be the first-of-its-kind cochlear implant designed to be fully implanted and use the ear, rather than a microphone, to pick up sound.
Interested readers can view a recent interview about the clinical trial below:
The implantation surgery was conducted by Colin Driscoll, MD, practicing neurotologist, professor of otolaryngology – head and neck surgery at Mayo Clinic, and principal investigator for this study. After a healing period, the Acclaim Cochlear Implant will be activated by Aniket Saoji, PhD, associate professor of otolaryngology – head and neck surgery at Mayo Clinic and co-investigator of the study. Both investigators serve on Envoy Medical’s Cochlear Implant Advisory Board.
“We at Envoy Medical are extremely proud that our Acclaim early feasibility study is taking place at Mayo Clinic. There is a strong medical device history and culture here in Minnesota, and we are honored to add to that legacy. This study is an important step towards demonstrating whether the fully implanted Acclaim Cochlear Implant works as it was designed, turning the page to a new chapter in cochlear implants. We hope to change the status quo within the hearing industry, and I am so proud of our team for bringing this breakthrough technology to fruition—implantable medical devices are not for the faint of heart.”
–Brent Lucas, CEO of Envoy Medical
Based on an Investigational Device Exemption (IDE) granted by the U.S. Food and Drug Administration (FDA), the early feasibility study for the Acclaim is expected to last 18 months and will be followed by a pivotal trial to support a Premarket Approval (PMA) application to the FDA.
Of the estimated 1.4-4 million adults with significant hearing loss in the U.S. who could benefit from cochlear implants, only roughly 5 percent of eligible adult candidates use cochlear implants.1 Potential adult recipients often wait longer than they should to get existing partially implanted cochlear implants, and a reason often cited is the amount of external hardware.2
Patients interested in learning more about the study should contact Amy Pajula, customer experience manager, at apajula@envoymedical.com for more information.
About the Fully Implanted Acclaim® Cochlear Implant
The fully implanted Acclaim® Cochlear Implant is a first-of-its-kind cochlear implant designed to address the hearing of adults diagnosed with severe to profound sensorineural hearing loss.
The Acclaim Cochlear Implant is designed to leverage unique sensor technology from the fully implanted Esteem active middle ear implant. Intended to address the limitations of current microphone-based hearing devices, Envoy Medical’s fully implanted technology includes a completely unique sensor designed to leverage the natural anatomy of the ear instead of a microphone to capture sound. The Esteem was FDA-approved in 2010.
The Acclaim Cochlear Implant received the Breakthrough Device Designation from the FDA. If approved by the FDA, the Acclaim would be the first-of-its-kind cochlear implant designed without any externally worn components and to use the ear to pick up sound.
CAUTION The fully implanted Acclaim Cochlear Implant is an investigational device. Limited by United States law to investigational use.
Important safety information for the Esteem can be found at: https://www.envoymedical.com/safety-information.
About Envoy Medical Corporation
Envoy Medical Corporation, headquartered in White Bear Lake, Minnesota, is a privately held hearing health company focused on providing innovative technologies across the hearing loss spectrum. Envoy Medical has pioneered one-of-a-kind, fully implanted devices for hearing loss, including its fully implanted Esteem® active middle ear implant, commercially available in the U.S. since 2010, and the fully implanted Acclaim® Cochlear Implant, an investigational device. Envoy Medical is dedicated to pushing hearing technology beyond the status quo to improve access, usability, compliance and ultimately quality of life. For more information, please visit www.envoymedical.com
Source: Envoy Medical






Sounds great. I assume it must use a battery? How is it charged?