Full Episode Transcript
Brian Taylor 0:10
Hello and welcome to another episode of This Week in hearing. I’m Brian Taylor. Our topic this week is cochlear implants and with us today to discuss Envoy Medical’s early feasibility study of their new Acclaim and CI is their CEO Brent Lucas, welcome to This Week in Hearing, Brent.
Brent Lucas 0:28
Thank you. Pleasure to be here. Thanks for having us.
Brian Taylor 0:30
Well, for most of our listeners out there, I think that who are in the hearing care professions, they’re most of them were probably not too familiar with Envoy Medical. So I thought maybe a good first question would be tell us a little bit about Envoy Medical.
Brent Lucas 0:47
Sure. So envoy medical is about a 25 year old startup company. It’s based in White Bear Lake, we’re Minnesota company, a lot of employees and investors have been from the Minnesota and Wisconsin area. St. Croix Medical was what our name was, for the first six or seven years. And before that, there was a group called Madison Devices Group that was involved with project, then they just ran up against the struggles of trying to develop a medical device that needs FDA approval and and found some folks, Ted Adams, and Ron Gooden were involved in the very beginning and then companies sort of took off from there.
Brian Taylor 1:38
Okay, well, I think I think some of our listeners will be familiar with St. Croix Medical, so it’s good to know about the recent name change. Yeah, yep. Well, the reason we have you here today, Brent is to talk about the Acclaim cochlear implant. I saw the press release looks really intriguing. Tell our audience a little bit about what makes it unique.
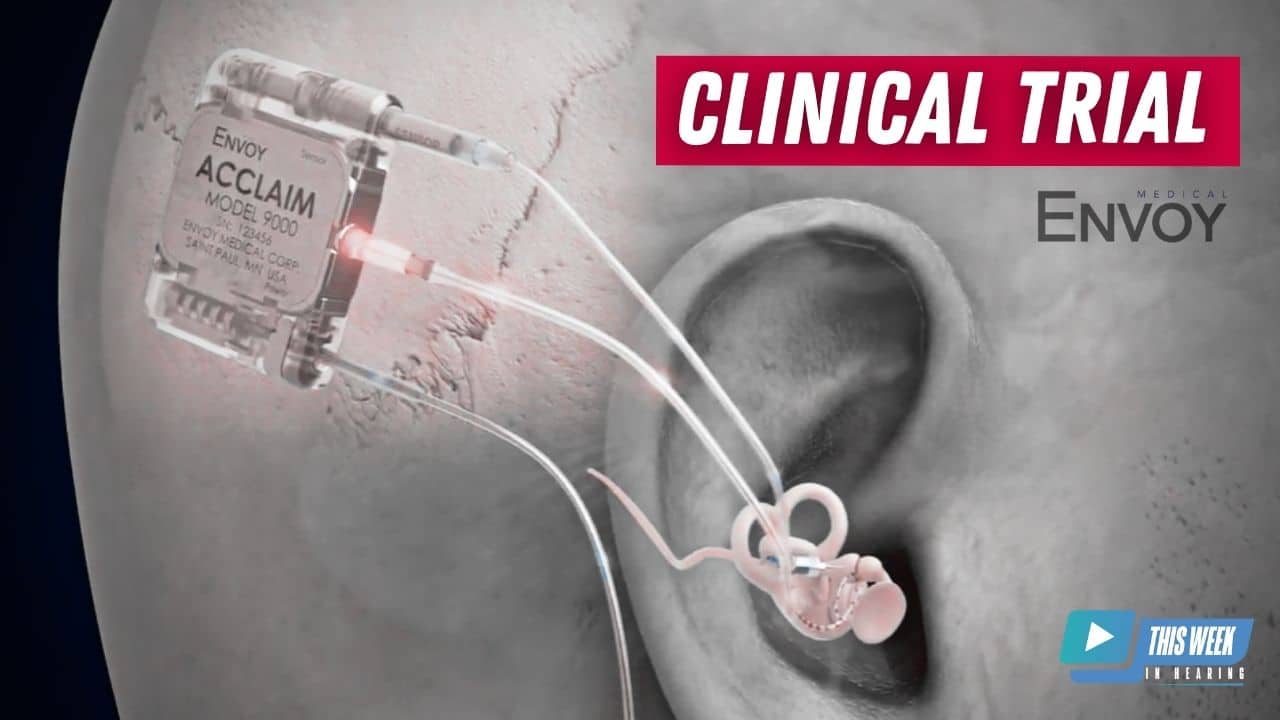
Brent Lucas 2:01
Sure. The Acclaim cochlear implant is different from what’s currently on the market in that it’s fully implanted. It is a fully implanted cochlear implant. So the the mechanism of operation if you will, is the same as you would expect from a cochlear implant there is electrical stimulation of the hearing nerve via the cochlea. The big difference for our device is that we use a sensor a piezoelectric sensor, similar to what’s in our Esteem device, our first middle ear device, we use that to pick up sound that comes in through the ear, and we essentially use the ear as the microphone versus an external behind the ear, microphone sound processor. We also believe we’re different from some of the other approaches to a fully implanted cochlear implant, in that we do not have an subdermal microphone, sort of a traditional microphone. And we also are going to have the device fully implanted throughout the day. So you will not have an external device charging it for portions of the day. This will be a truly a fully implanted device. Yeah, that’s pretty interesting.
Brian Taylor 3:11
I’m sure a lot of people are going to be wanting to know more about that. I wanted to kind of dive in a little bit and talk about the early feasibility study. I know that one of the neuro-otologists at Mayo Clinic in Rochester right down the road from both of us. Dr. Colin Driscoll is involved in that early feasibility study. Could you tell us a little bit about what you’re trying to accomplish with that?
Brent Lucas 3:36
Yes. So for folks who are familiar with class III implantable devices, you probably know that first in human trials, in the past were done in other countries and Europe or parts of Asia, maybe south of the border. The FDA started a program called the Early feasibility study program. I believe I have the title, right. But I know it’s an early feasibility study program and that they were trying to incentivize American companies and other companies from doing their first in human here in the in the United States, sort of identifying and understanding that we had a desire to get some early human data, especially for a device like ourselves, you can implant a cochlear implant into a animal for per se and say, you know, how does that sound and you can’t learn everything that you would like to learn from bench. So a first inhuman feasibility study is incredibly important to make sure we’re on the right track. And that the FDA has this program, early feasibility study program that we then are utilizing to get our first in human data points. And so Mayo Clinic like said in our backyard, just happens to have one of the best cochlear implant programs in the world. And Colin Driscoll is one of the best neuro otologist that you’ll come across very well respected. Not a cheerleader so to speak. So he’s going to tell it how it is. And then we also work with Anneka Soji, who is going to be the programming CI audiologist for our trial, who’s also incredibly steeped in cochlear implant industry was was actually on the, on the dark side and in industry for many years, and now is on the clinical side full time. So you
Brian Taylor 5:30
get some real heavy hitters involved in the project, which is great. Great to now.
Brent Lucas 5:34
Ya know, we have we have a wonderful, sorry to interrupt you, But we have a wonderful advisory board. We call it the cochlear implant advisory board. We have folks from Stanford, Washington University, St. Louis, we have some private practice individuals Lahey clinic. So we’ve really tried to get some of like said, heavy hitters, but people that are also going to make sure that we’re on the right path.
Brian Taylor 5:56
Always important with something like this, right. Assuming that the feasibility study goes, well, can you tell us a little bit more about who might be a candidate for the Acclaim CI
Brent Lucas 6:09
Sure, the the early feasibility study indications are what you would expect from sort of the traditional CI, indications in that it’s profound or severe to profound I should say, hearing loss. I know the industry is moving up to moderate to profound in terms of their indications. But we are, you know, we’re focused on sort of demonstrating to the FDA that we’re safe before they let us move up. The idea being that we will follow the trend of the industry and going to, you know, moderate to profound as we as we go through our pivotal trial. But the the indications are severe to profound, and then your typical, you know, 40% speech discrim sort of line of, they have to have worse hearing then that they can’t be deafened for too long period of time. Because we want to make sure that we’re actually testing out the implant works and not up against some of the natural atrophy of a brain that’s been, you know, unstimulated for many decades. So I would say no, you can’t obviously can’t have a lot of benefit from hearing aid. So just very typical cochlear implant indications. And then you’ll need to, you know, have general health of being able to undergo anesthesia, general anesthesia, as well, as you know, no contraindications related to the the implant itself. We, as I mentioned, it’s a fully implanted device, we didn’t really talk about the fact that the battery pack will be in the in the chest. And so if you have other hardware in there, right, now, we’re going to say you’re contraindicated so we don’t we don’t cross waves, so to speak.
Brian Taylor 7:53
Good to know. Is it accurate to say that the Acclaim would be for adults and not pediatrics?
Brent Lucas 8:02
Correct. Sorry. That’s a big point. Yes. This is for adults only for the early feasibility study and expect that to be the case for this version of the device going forward, something to look forward to down the road. But that’s right. That’s right.
Brian Taylor 8:17
I wanted to ask you, I know a few. We’ve had a few episodes here on the channel, talking about the relatively low uptake of CIs among adults, the fact that many of them go into see their hearing care professional and they don’t even know that they’re a candidate for a cochlear implant. And, you know, as you already kind of mentioned, the candidacy requirements over the last few years for CIs have broadened in adults. Could you speak maybe more generally, to how the profession can raise awareness in adults? The event the availability and the benefits from cochlear implants?
Brent Lucas 8:56
Sure, I I’d love to I have some thoughts that some are probably a little controversial. You know, you can’t you can’t talk to an industry that covers 40 million people without rubbing a few of them the wrong way. I would say that there’s a couple of things. One is I’m sort of a and I think, when I say I Envoys sort of an insider-outsider, if you will, we’re in the hearing industry, but nobody really has embraced middle ear implants. There’s a lot of skeletons on the side of the road of the hearing industry, and many of them are middle ear implants. And a large reason if not, the only reason is that it’s not covered by insurance, middle ear implants are not broadly reimbursed. And it’s hard to get market acceptance for a product that is not reimbursed. It’s hard for an audiologist, as much as they may want to recommend that product. It’s hard for them to think, Boy, how’s the patient going to pay for this? How are they going to have the care their long term, how is this? How am I going to be reimbursed, how’s the surging going to be reimbursed? So that is a significant problem. And I would say, and this is where it gets a little controversial, I would say that’s at the feet of the profession, I think audiologists and surgeons need to push for hearing loss to be taken more seriously by the the broad politicians, the societies and everything like that. I think the big manufacturers making all sorts of friends today, but I think the big, the big manufacturers have a vested interest in keeping the status quo. There’s not a lot of desire to see small companies like envoy or some of the others to get a foothold and and the reason for that is because they make a lot of money in the status quo, there’s, you know, it doesn’t take an MBA to understand why they don’t want things to change. So if there’s going to be change, it’s going to have to come from audiologists, and it’s going to have to come from the societies and professions that represent audiology and surgeons. So changing subjects a little bit in terms of adult CI candidates. Yes, the penetration is incredibly low. If you look at some of the publications on the matter, if you’re at talks and listen to folks, you’ll hear numbers between 3% penetration for adults, the eyes up to 10. So sort of the book ends are still incredibly low. We say 5%, we’ve done some market research to suggest it’s right around 5%. I think there’s the obvious reason, is there stigma around cochlear implants. Folks do not want a lot of the folks that we’ve talked to do not want to have the externals, either for stigma or quality of life. I think that’s a real concern. I know it’s not necessarily PC to say that, that there is folks who want an invisible device, but they there are folks who want an invisible device, you ask a surgeon or an audiologist one on one, what’s your biggest complaint, and they say it’s the externals, people don’t want that I pull up the hardware, I put it on the table, and they say they don’t want. So I think that’s it, I think the other is, is that there are a lot of competing interests within the hearing industry. There’s the quote, unquote, hearing aid audiologists, and then you have your implant audiologists, and there hasn’t been a great synergy between everybody involved, I know that people are working on that. And that’s not to paint with broad strokes. But there are definitely ways to improve, bringing everybody under the tent. And I I’m just a big believer in the fact that you know, the the old adage, high tides raise all ships, and I think if we’re gonna, if we really want the hearing industry to be what it can be, you need competition, you need innovation, and we all need to work together otherwise. And not to go on too much of a weird tangent. But I think you’re gonna see some of like, the brain interface companies and some other people go after the hearing industry. Because if you can just bypass the ear all together and go right to the brain. Why not? So I think the hearing industry really needs to, to adopt new technologies, or I think they’re, they’re going to be up against it in the future.
Brian Taylor 13:24
Yeah, no, I think that’s all really well said. And for all you hearing care professionals, audiologist hearing instrument specialists out there listening, you know, based on my 30 years experience, as an audiologist, I know that in your clinic, there are probably a couple of patients that are not doing as well as we want them to with their hearing aids that need to know about devices like the acclaim CI to CIs in general. So if you’re out there, please get familiar with the new candidacy requirements, find out where you can refer these patients for implants for a workup I think it’s really important to kind of broaden your scope of of care.
Brent Lucas 14:05
Yeah, and I don’t know when this is going to air but I know there’s still some comment period on the CMS proposed guidelines. And I think that’s been something that Terry Zolan and Craig Buckman have worked on for many, many years. And I started when John DeParco, I believe was was still alive. And you should voice your support for that because that is is helpful. And there’s also the the audiologists reimbursement act. That’s I don’t know remember the title for it. But you know, there’s a lot of things that can be pushed for it. It’s a huge market and we should we should really try to take care of ourselves so that we can take care of patients.
Brian Taylor 14:43
Yeah exactly and Bent thanks for all the insights final question or final thing I’d like to learn from you Brent, and that is, if people want to know more about the Acclaim feasibility study if they want to know more about Envoy Medical, do you have a web sight where can people learn more?
Unknown Speaker 15:01
Yeah, I appreciate that. envoymedical.com is our website. And we have some social media presence on Facebook and LinkedIn, and Twitter. And so find us on there. I don’t remember our handles off the top of my head but envoy medical will probably be a good, good way to find us. And then if you’re interested in early feasibility study, you should reach out directly to if you’re if you’re a patient or potential candidate, you should reach out directly to Mayo Clinic they handle all of that. And envoy is is not not involved in that. And then if you want to just know more about envoy in general, we have a great customer experience manager. Her name is Amy Patula, and she is a bilateral Esteem patient and so feel free to call her and talk to her about the device, either device and our patient experience.
Brian Taylor 15:59
It’s good to know Brent Lucas, CEO of Envoy Medical talking with us today about the Acclaim CI and the early feasibility study that they’re undergoing with the folks at Mayo Clinic in Rochester. Thanks again, Bremnt, for joining us. We appreciate it.
Brent Lucas 16:13
Thank you for your interest and I really appreciate the time
Be sure to subscribe to the TWIH YouTube channel for the latest episodes each week and follow This Week in Hearing on LinkedIn and Twitter.
Prefer to listen on the go? Tune into the TWIH Podcast on your favorite podcast streaming service, including Apple, Spotify, Google and more.
About the Panel
 Brian Taylor, AuD, is the senior director of audiology for Signia. He is also the editor of Audiology Practices, a quarterly journal of the Academy of Doctors of Audiology, editor-at-large for Hearing Health and Technology Matters and adjunct instructor at the University of Wisconsin.
Brian Taylor, AuD, is the senior director of audiology for Signia. He is also the editor of Audiology Practices, a quarterly journal of the Academy of Doctors of Audiology, editor-at-large for Hearing Health and Technology Matters and adjunct instructor at the University of Wisconsin.
 Brent Lucas is the Chief Executive Officer of Envoy Medical Corporation, based in White Bear Lake, Minnesota.
Brent Lucas is the Chief Executive Officer of Envoy Medical Corporation, based in White Bear Lake, Minnesota.