WHITE BEAR LAKE, MINNESOTA — A recent clinical publication in the Journal of Clinical Medicine (JCM) highlighted early surgical experience with Envoy Medical®, Inc.’s investigational Acclaim® fully implantable cochlear implant.
According to the publication:
“All three surgeries proceeded without complication, and at activation, all three patients were hearing through their devices. Surgery is more technically challenging compared to a standard cochlear implant, but the skills needed can be mastered by a dedicated otologic surgeon.”
The company currently expects to file for an investigational device exemption (IDE) in early 2024 to commence a pivotal clinical trial later that year.
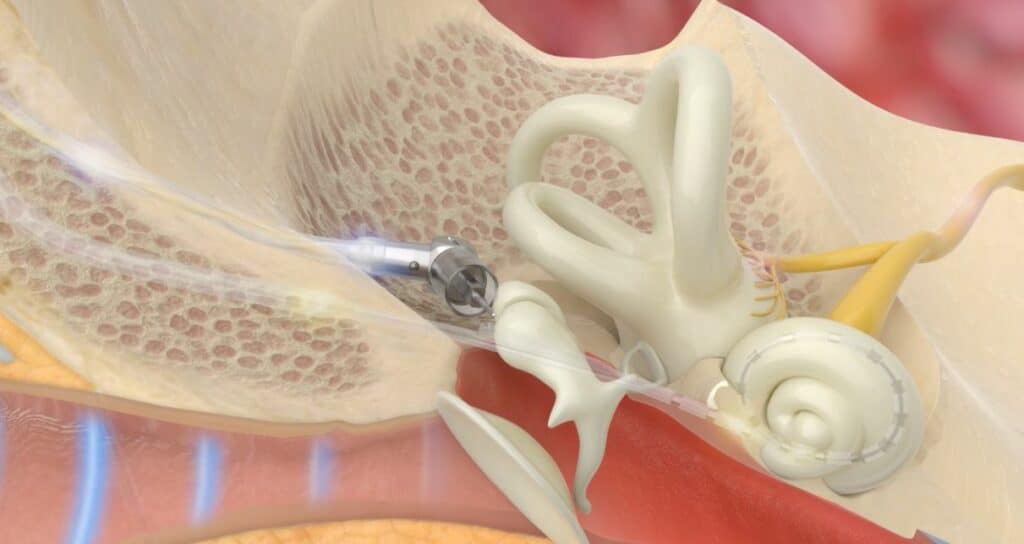
Fully Implanted Cochlear Implant Trial
Current cochlear implants rely on external hardware that are held in place on the head by a magnet, which can cause discomfort and may not be suitable for all activities, including sleeping, showering, swimming, strenuous activity and other common daily events.
Envoy Medical’s device, the fully implanted Acclaim® cochlear implant seeks to alleviate these limitations by being the first fully implanted cochlear implant (sometimes referred to as a totally implanted cochlear implant or “TICI”). By using an implanted middle ear sensor and an implanted rechargeable power supply, Acclaim® aims to eliminate the need for any externally worn components.
Acclaim® was granted Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) and is currently in an early feasibility study at Mayo Clinic (Rochester, Minnesota).
Envoy Medical’s Acclaim® may become the first fully implanted cochlear implant to begin a pivotal trial in the United States. Unlike other cochlear implants, Acclaim® intends to leverage the patient’s natural ear – rather than an external microphone – to capture acoustic energy. The Acclaim® is designed to not require the use of an external processor during the day or daily recharging.
“We are excited to see the Mayo Clinic’s team publish on their initial experience with the fully implanted Acclaim® cochlear implant, which we believe will be a significant advancement in a growing but underserved hearing health market. By detailing actual surgical experience, the authors provide cochlear implant specialists an early opportunity to learn about this innovation, the potential quality of life benefits of a fully implanted device, and that the additional surgical steps required likely will not be an impediment to adoption with appropriate training.”
–Brent Lucas, Envoy Medical CEO
“People often refuse to acknowledge the limitations of the current cochlear implants on the market in public, but in private, they do,” said CEO, Brent Lucas. “We believe people want a fully implanted cochlear implant, and we are excited to be pushing the industry in that direction just as companies like Inspire Medical pushed the sleep apnea industry towards a fully implanted solution.”
The Mayo Clinic authors note: “While it is only conjecture at this point, one may presume that increased breadth and comfort of use may improve quality-of-life for many recipients, particularly those who feel their disability prevents them from taking part in certain activities.”
The authors also highlight that this novel device is still developing. Certain patients may not be good candidates and that programming requires careful monitoring.
Underserved Market
If approved by the FDA, Envoy Medical intends to target a significantly under-penetrated adult cochlear implant market, which it believes to be more than $80 billion in the US. The paper notes that the success of cochlear implants has resulted in steadily widening the criteria by which patients can qualify for a cochlear device.
The complete study can be found under the citation:
- Dornhoffer, J.R.; Lawlor, S.K.; Saoji, A.A.; Driscoll, C.L.W. Initial Experiences with the Envoy Acclaim® Fully Implanted Cochlear Implant. J. Clin. Med. 2023, 12, 5875. https://doi.org/10.3390/jcm12185875
Interested readers can view a recent interview with company CEO, Brent Lucas discussing the clinical trial update below:
About Envoy Medical
Envoy Medical, Inc. (NASDAQ: COCH), headquartered in White Bear Lake, Minnesota, is a hearing health company focused on providing innovative medical technologies.
Envoy Medical is dedicated to pushing hearing technology beyond the status quo to provide patients with improved access, usability, independence and quality of life.
About the Fully Implanted Acclaim® Cochlear Implant
We believe the fully implanted Acclaim Cochlear Implant will be a first-of-its-kind cochlear implant. Envoy Medical’s fully implanted technology includes a sensor designed to leverage the natural anatomy of the ear instead of a microphone to capture sound.
The Acclaim is designed to address severe to profound sensorineural hearing loss that is not adequately addressed by hearing aids. The Acclaim is expected to be indicated for adults who have been deemed adequate candidates by a qualified physician.
The Acclaim Cochlear Implant received the Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) in 2019. We believe the Acclaim was the first hearing-focused device to receive Breakthrough Device Designation and may still be the only hearing focused medical technology to receive the designation.
CAUTION The fully implanted Acclaim Cochlear Implant is an investigational device. Limited by United States law to investigational use.
Important safety information for the Esteem can be found at: https://www.envoymedical.com/safety-information.
Source: Envoy Medical






