LOS ANGELES, CALIFORNIA — The capacity for hearing recovery remains elusive in adults with hearing loss due to the non-regenerative nature of the sensory hearing cells within the inner ear. Shedding light on this intricate phenomenon, University of Southern California (USC) Stem Cell scientists have undertaken two recent studies, partially supported by the National Institutes of Health and published in the Proceedings of the National Academy of Sciences (PNAS), which elucidate the underlying reasons for this limitation and propose potential avenues for transformative change.
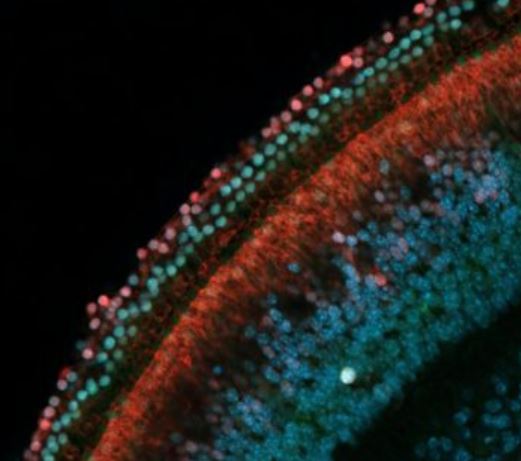
Rows of sensory hearing cells (green) next to supporting cells (red) in the inner ear of a mouse (Image by John Duc Nguyen and Juan Llamas/Segil Lab)
The two studies, conducted by a team of dedicated researchers including former students of the late Neil Segil, illuminate the intricate processes governing the transformation of non-sensory supporting cells into sensory hearing cells, thus paving the way for innovative strategies to reignite auditory functions in those deprived of this essential sense.
The first study, authored by John Duc Nguyen and his colleagues, delved into the critical role of epigenetic silencing in halting the regenerative potential of inner ear cells. Nguyen, who completed his research under the tutelage of Neil Segil, focused on the influence of chemical compounds known as methyl groups.
“In the non-sensory supporting cells of the inner ear, key genes required for conversion to sensory cells are shut off through a process known as ‘epigenetic silencing.’ By studying how the genes are shut off, we begin to understand how we might turn them back on to regenerate hearing”
–John Duc Nguyen, PhD
These compounds attach to DNA, rendering it inaccessible and effectively silencing the genes that orchestrate the conversion of non-sensory cells into sensory hearing cells. The team demonstrated the efficacy of an enzyme called TET in removing these methyl groups from DNA, thus reactivating the silenced genes and revitalizing the ability of supporting cells to transform into sensory hair cells.
In a remarkable twist, the researchers observed that gene silencing in supporting cells from chronically deafened mice was partially reversed, suggesting the intriguing possibility that the loss of sensory hearing cells could trigger a natural process of gene reactivation. This discovery holds the potential to spark a paradigm shift in our understanding of hearing restoration, offering hope for individuals grappling with auditory impairment.
Role of Sox4 and Sox11 in the Inner Ear
The second study, spearheaded by Emily Xizi Wang, uncovered the pivotal role of two genes, Sox4 and Sox11, in dictating the transition of inner ear progenitor cells into sensory hearing cells.
These genes, deemed essential for the formation of sensory hearing cells during development, presented a promising avenue for potential therapeutic interventions. By identifying the precise developmental window during which progenitor cells acquire the capacity to respond to the master regulator gene Atoh1, the team unveiled a crucial milestone in the path to auditory restoration.
“We focused on the genes Sox4 and Sox11 because we found that they are necessary for forming sensory hearing cells during development”
–Emily Xizi Wang, PhD
Intriguingly, mice lacking Sox4 and Sox11 exhibited a marked failure in the development of sensory hearing cells, underscoring the significance of these genes in the auditory regeneration process. Conversely, heightened activity of Sox4 and Sox11 yielded promising outcomes in both Petri dish experiments and mice with damaged sensory cells, showcasing a substantial increase in the conversion of supporting cells into sensory receptor cells.
Gage Crump, a co-author on both papers and the interim chair of USC’s Department of Stem Cell Biology and Regenerative Medicine at the Keck School of Medicine of USC, commented: “These two papers are not only great science, but also a clear example of Neil Segil’s enduring legacy as an exceptional mentor to the next generation of stem cell researchers.”
Further Reading:
- John D. Nguyen, Juan Llamas, Tuo Shi, J. Gage Crump, Andrew K. Groves, Neil Segil. DNA methylation in the mouse cochlea promotes maturation of supporting cells and contributes to the failure of hair cell regeneration. Proceedings of the National Academy of Sciences, 2023; 120 (33) DOI: 10.1073/pnas.2300839120
Source: USC, PNAS







