Hearing Aid Evolution – A Ride Through History
This post will review implantable hearing devices in this hearing aid evolution ride through history. In what has now become a six-part series, the first week focused on factors that drive hearing aid development, and then highlighted some of the major developments (primarily hardware) that shaped the changes to hearing aid design. The second post took the ride from power supplies through developments related to hearing aid transducers. The third post of the series reviewed changes that related more to developments of hearing aid fitting issues, and last week’s post featured hearing aid fitting directions.
Implantable Devices
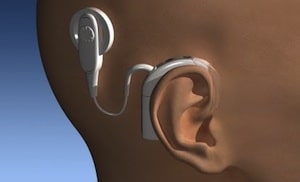
- Cochlear Implant – William House, M.D., is considered the “father of cochlear implants.” In 1961, he surgically performed his first cochlear implants after reading about earlier attempts in the late 1950’s in France. However, House’s implant was rejected by the patients’ bodies and had to be removed surgically. Following the introduction of biocompatible materials for other surgical procedures, Dr. House developed a longer lasting model and successfully implanted it in 1969. In 1972, a speech processor was developed to interface with the House 3M single-electrode implant, and in 1974, he implanted a single electrode into the cochlea (Figure 1). This was highly controversial at the time, but more than 1,000 of these devices were implanted in adults into the mid-1980s. This was the first cochlear implant device to be commercially marketed. In 1980, the age criteria for use of this device were lowered from 18 to 2 years. During the 1980’s, several hundred children were implanted with the House 3M single-channel device. By the late 80’s, virtually all the major concerns about the long-term success and safety of cochlear implants had been largely resolved.
Interestingly, it was not until 1977, more than a decade after the first House 3M single-electrode implant, that the Food and Drug Administration approved the cochlear implant, although specifically denouncing Dr. House’s version in favor of more complicated cochlear implant versions that were under development at that time and which later became the standard of use. When the American Academy of Ophthalmology and Otolaryngology (AAOO) endorsed implants in 1977, it too denounced Dr. House’s version.
The FDA formally approved the marketing of the 3M/House cochlear implant in November 1984. And during all this time (27 years), Dr. House faced stern opposition. His colleagues told him initially that cochlear implants would not work, or certainly, not very well. Others called it a cruel hoax on people desperate to hear, and some indicated he did this for financial gain (he never patented the cochlear implant because he did not want to discourage others from working on such devices). Some deaf advocates adamantly opposed the cochlear implant, saying that it would deprive its users of the dignity of their deafness without fully integrating them into the hearing world.
Part of the controversy related to the use of a single versus multiple electrodes to be inserted into the cochlea. House built and attempted a five-electrode device, but determined that overall performance was as well served with a single short electrode (House/3M cochlear implant) that stimulated the entire cochlea at once, required less total electrical energy, and promised to better preserve residual hearing. His interest continued in helping children hear better worldwide, especially in developing countries. He remained an advocate of a less expensive single-channel cochlear implant and developed a Vocal Hearing Screening Test to be conducted by the mother in the hospital or in well baby clinic visits. These works were done through his AllHear Company. He and I (post author) met various times to discuss these developments. In 2000, upon receiving a Life Achievement Award from the American Auditory Society, he acknowledged the numerous arrows in his back from all the various attacks. Dr. House died December 7, 2012.
Cochlear implants (CI) are now an established treatment for individuals with hearing that is essentially un-aidable using traditional hearing aids.
Today’s cochlear implants follow the same basic philosophy as the initial House implant, but are much more sophisticated (many more electrodes, programming schemes, miniaturization of components, complexity of function, reliability, digital signal processing, etc.). Figure 2 shows one such device. Current cochlear implant devices are manufactured by Cochlear Americas and Med-El.
- Bone-anchored Hearing Aids – Skin-penetrating hearing implants have been available since 1977. They have proven performance and advantages for patients with aural atresia, chronic ear drainage who cannot wear air-conduction hearing aids, conductive hearing losses, unilateral hearing loss, single sided deafness, and for people with mixed hearing losses who cannot otherwise wear traditional air-conduction hearing aids.
As with a number of developments in hearing assistance products, ideas were generated from work in other disciplines – in this case, dental and craniofacial reconstructive surgery. Osseointegration was using commercially pure titanium implants to support craniofacial reconstruction{{1}}[[1]] Branemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81–100[[1]]. Nine years later, Tjellstrom and his colleague{{2}}[[2]]Tjellstrom A, Granstrom G. One-stage procedure to establish osseointegration: a zero to five years follow-up report. J Laryngol Otol. 1995;109(7):593–598[[2]] introduced the concept of direct bone conduction, which could be achieved by using a skin-penetrating coupling from an osseointegrated titanium implant in the mastoid bone. The actual use of bone-anchored hearing aids as skin-penetrating implants began in 1977. The bone-anchored hearing concept became commercially available in 1987.
Historically, there have been both transcutaneous (over the skin) and percutaneous (through the skin) devices. A titanium-encased magnet is anchored to the mastoid bone (below the skin). The magnet is stimulated electromagnetically by an external energizing coil contained in a processor. The transcutaneous device, discontinued since about 1985, was designed by Hough and Xomed-Trease, and was called the Audiant. There were inherent problems with a transcutaneous approach because the transduced signal could be of low intensity, and in some cases the external device would not stay in contact with the skull{{3}}[[3]]Chasin, M. The future of implantable hearing devices: A special section (Part 1 of 2). Hearing Journal, August 2008, Vol. 61, Issue 8, pp 38,40[[3]].
The percutaneous design was developed by Nobel Biocare and was called the BAHA. This remains the only bone-anchored hearing aid on the market, and is now made by Cochlear Corp.
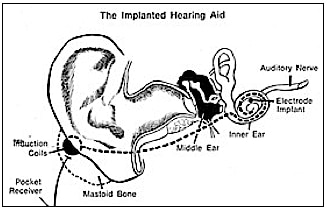
Bone-anchored hearing aids use a surgically implanted abutment to transmit sound by direct conduction through bone to the inner ear, bypassing the external auditory canal and middle ear (Figure 3). A titanium prosthesis is surgically embedded into the skull with a small abutment exposed outside the skin. A sound processor sits on this abutment and transmits sound vibrations to the titanium implant. The implant vibrates the skull and inner ear, which stimulate the nerve fibers of the inner ear, allowing hearing.
- Middle Ear Implant – 1996. A middle ear implant (MEI) is a hearing device, part or all of which is implanted in the middle ear. MEIs offer cosmetic advantages (they are not visible when worn), can provide greater high-frequency amplification because there is no interaction (acoustic feedback) between a microphone and receiver/speaker, produce no occlusion effect (own voice sounding loud and/or hollow), are active all the time because they are embedded, and, depending on the design, can be worn even when submerged in water with no damage.
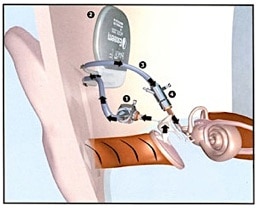
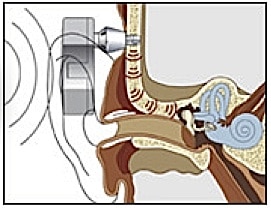
Figure 4. In this example of a middle ear implant (MEI), a surgical procedure implants an eardrum sensor in the middle ear cavity, takes that signal and amplifies it, sending the amplified signal to the cochlea via exaggerated movement of the auditory ossicles.
The first devices placed into the middle ear appeared in the 1970’s, even though they had been suggested earlier. The first MEI to come to market was in Japan. It was designed for patients with chronic dysfunction of the middle ear, and it replaced many of the middle ear’s functions. Possibly because of the complicated and irreversible nature of the surgery, this MEI approach did not gain widespread use. Later, a variety of approaches were tested, using different types of transducers to capture the signal and use the middle ear ossicles or the cochlear fluids as the transmission system. The transducers were primarily piezoelectric (bending and using the properties of piezoelectric materials), electromagnetic (magnet on the ossicles), or electromechanical (variation of electromagnetic in that the energizing coil and magnet are housed within an assembly with optimized spatial and geometric relationship to avoid inconsistent results).
In the example shown (Figure 4), a surgical procedure implants an eardrum sensor in the middle ear cavity, takes that signal and amplifies it, sending the amplified signal to the cochlea via exaggerated movement of the auditory ossicles. It does not use a microphone or speaker.
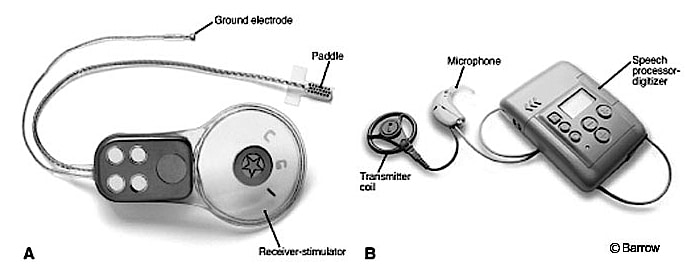
Figure 5. An auditory brainstem implant (ABI). This uses technology similar to a cochlear implant, but instead of electrical stimulation to stimulate the cochlea, it is used to stimulate the brain stem of the recipient.
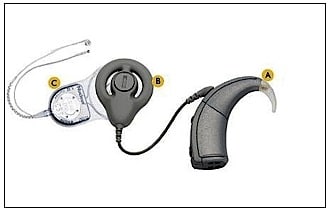
- Auditory Brainstem Implant – An early study by Simmons (1966){{4}}[[4]] Simmons, F. B. Electrical stimulation of the auditory nerve in man. Archives of Otolaryngology 84 (1): 2-54, 1966[[4]] placed electrodes through the promontory and vestibule directly into the modiolar segment of the auditory nerves. This demonstrated that in addition to being able to discern the signal duration, some degree of tonality could be achieved. An actual auditory brainstem implant (ABI) has its origins in the 1970’s at the House Ear Institute (HEI) in Los Angeles, CA (Dr. William House). The ABI (Figure 5) is a surgically implanted electronic device that provides a sense of sound to a profoundly deaf person. It uses technology similar to the cochlear implant, but instead of using electrical stimulation to stimulate the cochlea, it is used to stimulate the brainstem of the recipient. Brain surgery is required to implant the device. It has been shown to provide an awareness of sound. Its use remains rare.
Next week: Hearing Aid Coupling Evolution, Part VI.